Abstract
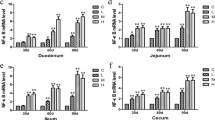
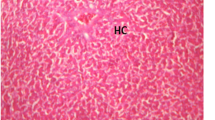
Arsenic (As) is a widely distributed trace element which is known to be associated with numerous adverse effects on human beings and animals. Arsenic trioxide (As2O3) is an inorganic arsenical-containing toxic compound. The effect of excessive amounts of As2O3 exposure on gastrointestinal tract tissue damage in cocks is still unknown. This study was conducted to investigate the effect of As2O3 exposure on gastrointestinal tract tissue damage in cocks. In total, 72 1-day-old male Hyline cocks were randomly divided into four groups and fed either a commercial diet or an As2O3 supplement diet containing 7.5, 15, and 30 mg/kg As2O3. The experiment lasted for 90 days and gastrointestinal tract tissue samples (gizzard, glandular stomach, duodenum, jejunum, ileum, cecum, and rectum) were collected at 30, 60, and 90 days. Catalase (CAT), glutathione (GSH), and glutathione peroxidase (GSH-Px) activities; malondialdehyde (MDA) contents; and hydroxyl radical (OH·)-mediated inhibition were examined. Furthermore, the results demonstrated that MDA content in the gastrointestinal tract was increased, while the activities of CAT, GSH, and GSH-Px and the ability to resist OH· was decreased in the As2O3 treatment groups. Extensive damage was observed in the gastrointestinal tract. These findings indicated that As2O3 exposure caused oxidative damage in the gastrointestinal tract of cocks due to alterations in antioxidant enzyme activities and elevation of free radicals.






Similar content being viewed by others
References
Squibb KS, Fowler BA (1983) The toxicity of arsenic and its compounds. In: Fowler BA (ed) Biological and environmental effects of arsenic, Chap 7. Elsevier Science Publishers, Amsterdam, pp 233–269
Arena JM, Drew RH (1986) Poisoning: toxicology, symptoms, treatments, fifth edition. Springfield, IL7 Charles C. Thomas, pp. 11–28
Kazi TG, Shah AQ, Afridi HI et al (2013) Hazardous impact of organic arsenical compounds in chicken feed on different tissues of broiler chicken and manure. Ecotox Environ Safe 87:120–123
Nordstrom DK (2002) Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
Cervantes C, Ji G, Ramirez JL et al (1994) Resistance to arsentic compounds in microorganisms. FEMS Microbiol Rev 15:355–367
Zaloga GP, Deal J, Spurling T et al (1985) Case report: unusual manifestations of arsenic intoxication. Am J Med Sci 289:210–214
Ahmad S, Kitchin KT, Cullen WR (2000) Arsenic species that cause release of iron from ferritin and generation of activated oxygen. Arch Biochem Biophys 382:195–202
Brochmoller J, Cascorbi I, Henning S et al (2000) Roots: I molecular genetics of cancer susceptibility. Pharmacol Res 61:212–227
Kozul CD, Ely KH, Enelow RI et al (2009) Low-dose arsenic compromises the immune system response to influenza A infection in vivo. Environ Health Perspect 117:1441–1447
Argos M, Kalra T, Rathouz PJ et al (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Jomova K, Vondrakova D, Lawson M et al (2010) Metals, oxidative stress and neurodegenetative disorders. Mol Cell Biochem 345:91–104
Gunter TE, Gavin CE, Aschner M et al (2006) Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicol 27(5):765–776
Joseph B, Jini D (2010) Insight into the role of antioxidant enzymes for salt tolerance in plants. Int J Bot 6(4):456–464
Kumari GJ, Reddy AM, Naik ST et al (2006) Jasmonic acid induced changed in protein pattern, antioxidative enzyme activities and peroxidase isozymes in peanut seedlings. Biol Plant 50(2):219–226
Lapointe J, Kimmins S, Maclaren LA et al (2005) Estrogen selectively upregulates the phospholipid hydroperoxide glutathione peroxidase in the oviducts. Endocrinol 146:2583–2592
Meloni DA, Oliva MA, Martinez CA et al (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Chen Y, Parvez F, Gamble M et al (2009) Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarker for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol 239:184–192
Webb JL (1966) Enzymes and metabolic inhibitors. Academic Press, New York, pp 595–793
Pi J, Yamauchi H, Kumagai Y et al (2002) Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ Health Perspect 110:331–336
Shi H, Hudson LG, Ding W et al (2004) Arsenite causes DNA damage in keratinocytes via generation of hydroxyl radicals. Chem Res Toxicol 17:871–878
Kitchin KT, Ahmad S (2003) Oxidative stress as a possible mode of action for arsenic carcinogenesis. Toxicol Lett 137:3–13
Gebel T (2000) Confounding variables in the environmental toxicology of arsenic. Toxicology 144:155–162
Xing HJ, Li S, Wang ZL et al (2012) Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 88:377–383
Xu SW, Yao HD, Zhang J et al (2013) The oxidative damage and disbalance of calcium homeostasis in brain of chicken induced by selenium deficiency. Biol Trace Elem Res 151:225–233
Zhu WJ, Li M, Liu C et al (2013) Avermectin induced liver injury in pigeon: mechanisms of apoptosis and oxidative stress. Ecotoxicol Environ Saf 98:74–81
Papp LV, Lu J, Holmgren A et al (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Sign 9:775–806
Zhang ZW, Wang QH, Zhang JL et al (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149:352–361
Liu CM, Zheng GH, Ming QL et al (2013) Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J Agr Food Chem 61(5):1146–1154
Pari L, Amudha K (2011) Hepatoprotective role of naringin on nickel-induced toxicity in male Wistar rats. Eur J Pharmacol 650(1):364–370
Misra M, Rodriguez RE, Kasprzak KS (1990) Nickel induced lipid peroxidation in the rat: correlation with nickel effect on antioxidant defense systems. Toxicology 64(1):1–17
Rao MV, Chawla SL, Sharma SR (2009) Protective role of vitamin E on nickel and/or chromium induced oxidative stress in the mouse ovary. Food Chem Toxicol 47(6):1368–1371
Lou J, Jin L, Wu N et al (2013) DNA damage and oxidative stress in human B lymphoblastoid cells after combined exposure to hexavalent chromium and nickel compounds. Food Chem Toxicol 55:533–540
Liu LL, Cheng ML, Zhang ZW et al (2014) Protective effects of selenium on cadmium-induced brain damage in chicken. Biol Trace Elem Res 158:176–185
Acknowledgments
This study was supported by the Postdoctoral Scientific Research Developmental Foundation of Heilongjiang Province (Grant No. LBH-Q13012). We thank American Journal Experts for assistance with this manuscript.
Conflict of Interest
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Ying Guo and Panpan Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, Y., Zhao, P., Guo, G. et al. The Role of Oxidative Stress in Gastrointestinal Tract Tissues Induced by Arsenic Toxicity in Cocks. Biol Trace Elem Res 168, 490–499 (2015). https://doi.org/10.1007/s12011-015-0357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0357-9