Abstract
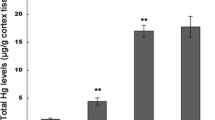
Methylmercury (MeHg) is a well-known environmental pollutant leading to neurotoxicant associated with aberrant central nervous system (CNS) functions, but its toxic mechanisms have not yet been fully recognized. In the present study, we tested the hypothesis that MeHg induces neuronal injury via glutamate (Glu) dyshomeostasis and oxidative damage mechanisms and that these effects are attenuated by dextromethorphan (DM), a low-affinity and noncompetitive N-methyl-d-aspartate receptor (NMDAR) antagonist. Seventy-two rats were randomly divided into four groups of 18 animals in each group: control group, MeHg-treated group (4 and 12 μmol/kg), and DM-pretreated group. After the 4-week treatment, we observed that the administration of MeHg at a dose of 12 μmol/kg significantly increased in total mercury (Hg) levels, disrupted Glu metabolism, overexcited NMDARs, and led to intracellular calcium overload in the cerebral cortex. We also found that MeHg reduced nonenzymatic and enzymatic antioxidants, enhanced neurocyte apoptosis, induced reactive oxygen species (ROS), and caused lipid, protein, and DNA peroxidative damage in the cerebral cortex. Moreover, glutamate/aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) appeared to be inhibited by MeHg exposure. These alterations were significantly prevented by the pretreatment with DM at a dose of 13.5 μmol/kg. In conclusion, these findings strongly implicate that DM has potential to protect the brain from Glu dyshomeostasis and oxidative damage resulting from MeHg-induced neurotoxicity in rat.










Similar content being viewed by others
References
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Aschner M, Syversen T, Souza DO et al (2007) Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40:285–291
Sirois JE, Atchison WD (2000) Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells. Toxicol Appl Pharmacol 167:1–11
Atchison WD (2005) Is chemical neurotransmission altered specifically during methylmercury-induced cerebellar dysfunction? Pharmacol Sci 26:549–557
Aschner M, Yao CP, Allen JW et al (2000) Methylmercury alters glutamate transport in astrocytes. Neurochem Int 37:199–206
Farina M, Dahm KC, Schwalm FD et al (2003) Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol Sci 73:135–140
Mutkus L, Aschner JL, Syversen T et al (2005) Methylmercury alters the in vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells. Biol Trace Elem Res 107:231–245
Ou YC, White CC, Krejsa CM et al (1999) The role of intracellular glutathione in methylmercury-induced toxicity in embryonic neuronal cells. Neurotoxicology 20:793–804
Manfroi CB, Schwalm FD, Cereser V et al (2004) Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci 81:172–178
Yin Z, Milatovic D, Aschner JL et al (2007) Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res 1131:1–10
Featherstone DE (2010) Intercellular glutamate signaling in the nervous system and beyond. ACS Chem Neurosci 1:4–12
Erikson K, Aschner M (2002) Manganese causes differential regulation of glutamate transporter (GLAST) taurine transporter and metallothionein in cultured rat astrocytes. Neurotoxicity 23:595–602
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276
Pivovarova NB, Andrews SB (2010) Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J 277:3622–3636
Lafon-Cazal M, Pietri S, Culcasi M et al (1993) NMDA-dependent superoxide production and neurotoxicity. Nature 364:535–537
Ceccatelli S, Dare E, Moors M (2010) Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact 188:301–308
Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14
Maragakis NJ, Rothstein JD (2001) Glutamate transporters in neurologic disease. Arch Neurol 58:365–370
Farina M, Aschner M, Rocha JB (2011) Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol 256:405–417
Farina M, Rocha JB, Aschner M (2011) Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89:555–563
Dreiem A, Seegal RF (2007) Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. NeuroToxicology 28:720–726
Alko M, Morris H, Cronin M (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Qiuyin C, Rahn RO, Ruiwen Z (1997) Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett 119:99–107
Franco JL, Braga HC, Stringari J (2007) Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol 20:1919–1926
Lucena GM, Franco JL, Ribas CM et al (2007) Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin Pharmacol Toxicol 101:127–131
Farina M, Campos F, Vendrell I et al (2009) Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci 112:416–426
Farina M, Soares FA, Zeni G et al (2004) Additive pro-oxidative effects of methylmercury and ebselen in liver from suckling rat pups. Toxicol Lett 146:227–235
Chang JY, Tsai PF (2008) Prevention of methylmercury-induced mitochondrial depolarization, glutathione depletion and cell death by 15-deoxy-delta-12, 14-prostaglandin J(2). NeuroToxicology 29:1054–1061
Roos DH, Puntel RL, Santos MM et al (2009) Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: involvement of oxidative stress and glutamatergic system. Toxicol Vitro 23:302–307
Allen JW, Mutkus LA, Aschner M (2001) Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res 902:92–100
Allen JW, Shanker G, Aschner M (2001) Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res 894:131–140
Bordji K, Becerril-Ortega J, Buisson A (2011) Synapses, NMDA receptor activity and neuronal Aβ production in Alzheimer’s disease. Rev Neurosci 22:285–294
Papadia S, Soriano FX, Fet L et al (2008) Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11:476–487
Zhang W, Wang T, Qin L et al (2004) Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J 18:589–591
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Stockwell PB, Corns WT (1993) The role of atomic fluorescence spectrometry in the automatic environmental monitoring of trace element analysis. J Autom Chem 15:79–84
Curi TC, Melo MP, Azevedo RB et al (1997) Glutamine utilization by rat neutrophils: presence of phosphate-dependent glutaminase. Am J Physiol 273:C1124–C1129
Ahamed M, Akhtar MJ, Siddiqui MA et al (2011) Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 283:101–108
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–311
Mori N, Yasutake A, Hirayama K (2007) Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol 81:769–776
Chan E, Yung WH, Baumann KI (1996) Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Exp Brain Res 108:357–366
Cheng WW, Lin ZQ, Wei BF et al (2011) Single-walled carbon nanotube induction of rat aortic endothelial cell apoptosis: reactive oxygen species are involved in the mitochondrial pathway. Int J Biochem Cell Biol 43:564–572
Guerguerian AM, Brambrink AM, Traystman RJ et al (2002) Altered expression and phosphorylation of N-methyl-d-aspartate receptors in piglet striatum after hypoxiaischemia. Brain Res 104:66–80
Yin Z, Jiang H, Syversen T et al (2008) The methylmercury-l-cysteine conjugate is a substrate for the l-type large neutral amino acid transporter. J Neurochem 107:1083–1090
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Juarez BI, Martınez ML, Montante M et al (2002) Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol 24:767–771
Carratu MR, Borracci P, Coluccia A et al (2006) Acute exposure to methylmercury at two developmental windows: focus on neurobehavioral and neurochemical effects in rat offspring. Neuroscience 141:1619–1629
Boulland JL, Osen KK, Levy LM et al (2002) Cell-specific expression of the glutamine transporter SN1 suggests differences in dependence on the glutamine cycle. Eur J Neurosci 15:1615–1631
Sidoryk-Wegrzynowicz M, Lee E, Albrecht J et al (2009) Manganese disrupts astrocyte glutamine transporter expression and function. J Neurochem 110:822–830
Rothstein JD, Martin L, Levey AI et al (1996) Localization of neuronal and glial glutamate transporters. Neuron 13:713–725
Nakamichi N, Ohno H, Kuramoto N et al (2002) Dual mechanisms of Ca2+ increases elicited by N-methyl-d-aspartate in immature and mature cultured cortical neurons. J Neurosci Res 67:275–283
Wu HY, Yuen EY, Lu YF et al (2005) Regulation of N-methyl-d-aspartate receptors by calpain in cortical neurons. J Biol Chem 280:21588–21593
Simpkins KL, Guttmann RP, Dong Y et al (2003) Selective activation induced cleavage of the NR2B subunit by calpain. J Neurosci 23:11322–11331
Wu HY, Hsu FC, Gleichman AJ et al (2007) Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem 82:20075–20087
Nel A, Xia T, Madler L et al (2006) Toxic potential of materials at the nanolevel. Virtual J Nanoscale Sci Technol 13:622–627
Stone V, Donaldson K (2006) Nanotoxicology: signs of stress. Nat Nanotechnol 1:23–24
Shanker G, Syversen T, Aschner JL et al (2005) Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Mol Brain Res 137:11–22
Farina M, Franco JL, Ribas CM et al (2005) Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol 57:1503–1508
Carvalho MC, Franco JL, Ghizoni H et al (2007) Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology 239:195–203
Halliwell B (1992) Oxygen radicals as key mediators in neurological disease: fact or fiction? Ann Neurol 32:S10–S15
Niki E, Yoshida Y, Saito Y et al (2005) Lipid peroxidation: mechanism, inhibition, and biological effects. Biochem Biophys Res Commun 336:1–9
Reynolds IJ, Hastings TG (1995) Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15(5 Pt 1):3318–3327
Sanz-Blasco S, Valero RA, Rodri guez-Crespo I et al (2008) Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS ONE 3:e2718
Swamy M, Salleh MJ, Sirajudeen KN et al (2010) Nitric oxide (no), citrulline - no cycle enzymes, glutamine synthetase and oxidative stress in anoxia (hypobaric hypoxia) and reperfusion in rat brain. Int J Med Sci 7:147–154
Carpenter CL, Marks SS, Watson DL et al (1988) Dextromethorphan and dextrorphan as calcium channel antagonists. Brain Res 439:372–375
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant no.81172631).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, S., Xu, Z., Liu, W. et al. Preventive Effects of Dextromethorphan on Methylmercury-Induced Glutamate Dyshomeostasis and Oxidative Damage in Rat Cerebral Cortex. Biol Trace Elem Res 159, 332–345 (2014). https://doi.org/10.1007/s12011-014-9977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-9977-8