Abstract
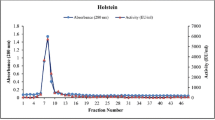
In this study, paraoxonase 1 (PON1; EC 3.1.8.1) was purified from bull semen, and some characteristics of the enzyme were investigated. In vitro inhibition effect of some heavy metals, including Cu2+, Mn2+, Cd2+, Zn2+, Ni2+, and Pb2+, on the activity of the purified enzyme was also investigated. The purification of bull semen PON1 procedure was composed of two steps: ammonium sulfate precipitation and Sepharose-4B-l-tyrosine-1-naphthylamine hydrophobic interaction chromatography. The enzyme, having a specific activity of 288 EU/mg proteins, was purified 22.67-fold with a yield of 89 %. Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of the purified enzyme showed the presence of a single band with an apparent MW of 66 kDa. The V max and K M values for the paraoxon substrate were determined as 100 EU and 8.0 × 10−5 M, respectively. The inhibitory effects of different heavy metals on PON1 activity were determined by using the paraoxon as a substrate. The results showed that all the metals, except for Cd2+, inhibited the PON1 enzyme activity in a concentration-dependent fashion. IC50 values of Cu2+, Mn2+, Zn2+, Ni2+, and Pb2+ were found as 2.59 × 10−3, 1.17 × 10−3, 42.74 × 10−3, 99.10 × 10−3, 48.80 × 10−3 mM, respectively. Conversely, Cd2+ increased the bull semen PON1 enzyme activity. The present study has demonstrated that Cu2+, Mn2+, Zn2+, Ni2+, and Pb2+ are serious toxic metals, which are able to increase the risk of oxidative stress development and a subsequent decrease of semen quality.




Similar content being viewed by others
References
Arslan M, Erzengin M, Demir D (2011) Comparison of serum paraoxonase 1 (PON1) activities among different sheep breeds in Turkey. J Anim Vet Adv 10(4):489–494
Mackness MI, Walker CH (1988) Multiple forms of sheep serum A-esterase activity associated with the high-density lipoprotein. Biochem J 250:539–545
Mackness B, Durrington PN, Mackness MI (1998) Human serum paraoxonase. Gen Pharmacol 31:329–336
Kokouvaa M, Koureasa M, Dardiotisb E, Almpanidoub P, Kalogerakic A, Kyriakoud D, Hadjigeorgioub GM, Hadjichristodouloua C (2013) Relationship between the paraoxonase 1 (PON1) M55L and Q192R polymorphisms and lymphohaematopoietic cancers in a Greek agricultural population. Toxicology 307:12–16
Khersonsky O, Tawfik D (2005) Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 44(16):6371–6382
Camps J, Marsillach J, Joven J (2009) The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci 46(2):83–106
La Du BN, Aviram M, Billecke S, Navab M, Primo-Parmo S, Sorenson RC, Standiford TJ (1999) On the physiological role(s) of the paraoxonases. Chem Biol Interact 119–120:379–388
Erzengin M, Basaran I, Cakir U, Aybey A, Sinan S (2012) In vitro inhibition effect of some dihydroxy coumarin compounds on purified human serum paraoxonase 1 (PON1). Appl Biochem Biotechnol 168:1540–1548
Aviram M, Rosenblat M (2004) Paraoxonases 1, 2, and 3, oxidative stress, and macrophage cell formation during atherosclerosis development. Free Radic Biol Med 37(9):1304–1316
Mackness MI (1989) Possible medical significance of human serum “A”-esterases. In: Reiner E, Aldridge WN, Hoskin FCG (eds) Enzymes hydrolysing organophosphorus compounds. Ellis Horwood, Chichester, pp 203–213
Mackness MI (1989) “A”-esterases: enzymes looking for a role? Biochem Pharmacol 38:385–390
Mackness MI, Durrington PN (1995) HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis 115:243–253
Stein O, Stein Y (1999) Atheroprotective mechanisms of HDL. Atherosclerosis 144:285–301
Hu H, McCally M (eds) (2002) Life support: the environment and human health (book review). J Sociol Soc Welf 4:1-9
Foote RH, Brockett CC, Kaproth MT (2002) Motility and fertility of bull sperm in whole milk extender containing antioxidants. Anim Reprod Sci 71:13–23
Stohs SJ, Baggihi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Med 18:321–336
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–251
Gan KN, Smolen A, Eckerson HW, La Du BN (1991) Purification of human serum paraoxonase/arylesterase, evidence for one esterase catalyzing both activities. Drug Metab Dispos 19:100–106
Sinan S, Kockar F, Arslan O (2006) Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglycoside derived antibiotics. Biochimie 88:565–574
Demir N, Nadaroğlu H, Demir Y (2008) Purification of human serum paraoxonase and effect of acetylsalicylic acid on paraoxonase activity in vitro and rat serum liver and heart in vivo. Pharm Biol 46(6):393–399
Laemmli DK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–683
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 57:685
Kara HE, Sinan S, Turan Y (2011) Purification of beta-glucosidase from olive (Olea europaea L.) fruit tissue with specifically designed hydrophobic interaction chromatography and characterization of the purified enzyme. J Chromatogr B 879:1507–1512
Samra ZQ, Shabir S, Rehmat Z, Zaman M, Nazir A, Dar N, Athar MA (2010) Synthesis of cholesterol-conjugated magnetic nanoparticles for purification of human paraoxonase 1. Appl Biochem Biotechnol 162:671–686
Pla A, Rodrigo L, Hernandez AF et al (2007) Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem Biol Interact 167:63–70
Erol K, Gençer N, Arslan M, Arslan O (2013) Purification, characterization, and investigation of in vitro inhibition by metals of paraoxonase from different sheep breeds. Artif Cells Blood Substit Biotechnol 41:125–130
Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, Paneth N, Wirth JJ (2008) Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive. Tox Environ Health Perspect 116(11):1473–1479
Gençer N, Arslan O (2009) Purification human PON1Q192 and PON1R192 isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chromatogr B 877:134–140
Ekinci D, Beydemir Ş (2010) Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 135:112–120
Verit FF, Verit A, Ciftci H, Erel O, Celik H (2009) Paraoxonase-1 activity in subfertile men and relationship to other sperm parameters. J Androl 30:183–189
Massanyi P, Trandzik J, Nad P, Korenekova B, Skalicka M, Toman R, Lukac N, Halo M, Strapak P (2004) Concentration of copper, iron, zinc, cadmium, lead, and nickel in bull and ram semen and relation to the occurrence of pathological spermatozoa. J Environ Sci Health A 39(11–12):3005–3014
Tvrda E, Knazıcka Z, Lukac N (2012) Selected heavy metals versus antioxidant parameters in bull seminal plasma—a comparative study. J Environ Sci Health A 47:1261–1266
Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS (1998) Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 59:1037–1046
Storey BT (1997) Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod 3:203–214
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors alone are responsible for the content and writing of the article.
Rights and permissions
About this article
Cite this article
Dedeoğlu, N., Arslan, M. & Erzengin, M. Purification of Holstein Bull Semen Paraoxonase 1 (PON1) by Hydrophobic Interaction Chromatography and Investigation of Its Inhibition Kinetics by Heavy Metals. Biol Trace Elem Res 158, 29–35 (2014). https://doi.org/10.1007/s12011-014-9916-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-9916-8