Abstract
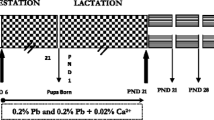
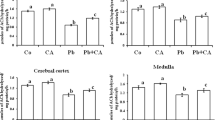
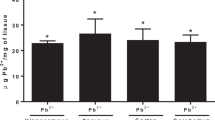
Epidemiological studies in children have proved that lead (Pb) exposure causes deficits in neural and cognitive functions. The present study assessed the oxidative stress on postnatal day 30, in the hippocampus, cerebellum and frontal cortex of rat pups exposed to Pb during specific periods of early brain development. Five groups of rat pups were investigated, and 0.2 % Pb acetate in drinking was the dosage used. (i) Gestation and lactation (GL) group (n = 9) of rat pups was exposed to Pb during gestation and lactation through their mother, (ii) gestation (G) group (n = 9) of rat pups was exposed to Pb during gestation only, (iii) lactation (L) group (n = 9) of rat pups was exposed to Pb during lactation only, (iv) pre-gestation (PG) group (n = 9) of rat pups was born to mothers who were exposed to Pb for 1 month before conception, and (v) normal control (NC) (n = 9) group of rats pups had no exposure to Pb during gestation and lactation period. From the present study, it is evident that Pb exposure during different periods of early brain development (GL, G, L and PG groups) causes oxidative stress and lactation period (postnatal period) of Pb exposure produces maximum oxidative stress.




Similar content being viewed by others
References
Yiin SJ, Lin TH (1995) Lead-catalyzed peroxidation of essential unsaturated fatty acid. Biol Trace Elem Res 50:167–172
Adegbesan BO, Adenuga GA (2007) Effect of lead exposure on liver lipid peroxidative and antioxidant defense systems of protein-undernourished rats. Biol Trace Elem Res 116:219–225
Bokara KK, Brown E, McCormick R et al (2008) Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. Biometals 21:9–16
Adonaylo VN, Oteiza PI (1999) Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology 135:77–85
Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O (1998) Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Mol Brain Res 53:333–338
Steullet P, Cabungcal JH, Kulak A et al (2010) Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci 30:2547–2558
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Goyer RA (1990) Transplacental transport of lead. Environ Health Perspect 89:101–105
Lataillade GP, Thoreux-Manlay A, Coffigny H, Masse R, Soufir JC (1995) Reproductive toxicity of chronic lead exposure in male and female mice. Hum Exp Toxicol 14:872–878
Namihira D, Saldivar N, Pustilnik N, Carreon GJ, Salinas ME (1993) Lead in human blood and milk from nursing women living near a smelter in Mexico city. J Toxicol Environ Health 388:225–232
Hallen PI, Jorhem L, Oskarsson A (1995) Placental and lactational transfer of lead in rats: a study of the lactational process and effects on offspring. Arch Toxicol 69:596–602
Needleman HL (1993) The current status of childhood low-level lead toxicity. Neurotoxicology 14:161–166
Markowitz M (2000) Lead poisoning: a disease for the next millennium. Curr Probl Pediatr 30:62–70
Gurer H, Ozgunes H, Neal R et al (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128:181–189
Lu X, Jin C, Yang J et al (2013) Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats' hippocampus. Biol Trace Elem Res 151(1):75–84
Muller RU, Bostock E, Taube JS, Kubie JL (1994) On the directional firing properties of hippocampal place cells. J Neurosci 14(12):7235–7251
Hayman R, Verriotis MA, Jovalekic A, Fenton AA, Jeffery KJ (2011) Anisotropic encoding of three-dimensional space by place cells and grid cells. Nat Neurosci 14(9):1182–1188
Holdstock JS, Mayes AR, Roberts N et al (2002) Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus 12:341–351
Griffiths D, Dickinson A, Clayton N (1999) Episodic memory: what can animals remember about their past? Trends Cogn Sci 3:74–80
Tulving E, Markowitsch HJ (1998) Episodic and declarative memory: a role of the hippocampus. Hippocampus 8:198–204
Raymond JL, Lisberger SG, Mauk MD (1996) The cerebellum: a neural learning machine? Science 272:1126–1131
Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M (1999) Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiat 46(7):908–920
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16(3):367–378
Kolb B (1990) Animal models for human PFC-related disorders. Prog Brain Res 85:501–519
Stuss DT, Alexander MP (2000) Executive functions and the frontal lobes: a conceptual view. Psychol Res 63:289–298
Winocur G, Moscovitch M (1999) Anterograde and retrograde amnesia after lesions to frontal cortex in rats. J Neurosci 19(21):9611–9617
Moreira EG, Rosa GJ, Barros SB, Vassilieff VS, Vassillieff I (2001) Antioxidant defense in rat brain regions after developmental lead exposure. Toxicology 169(2):145–151
Verma SK, Dua R, Gill KD (2006) Impaired energy metabolism after co-exposure to lead and ethanol. Basic Clin Pharmacol Toxicol 96(6):475–479
Barkur RR, Bairy LK (2014) Histological study on hippocampus, amygdala and cerebellum following low lead exposure during prenatal and postnatal brain development in rats. Toxicol Ind Health. doi:10.1177/0748233714545624
Jaako-Movits K, Zharkovsky T, Romantchik O et al (2005) Developmental lead exposure impairs contextual fear conditioning and reduces adult hippocampal neurogenesis in the rat brain. Int J Dev Neurosci 23:627–635
Heidmets LT, Zharkovsky T, Jurgenson M, Jaako-Movits K, Zharkovsky A (2006) Early postnatal, low-level lead exposure increases the number of PSA-NCAM expressing cells in the dentate gyrus of adult rat hippocampus. Neurotoxicology 27:39–43
Chen HH, Ma T, Paul IA, Spencer JL, Ho IK (1997) Developmental lead exposure and two-way active avoidance training alter the distribution of protein kinase C activity in the rat hippocampus. Neurochem Res 22:1119–1125
Glowinski J, Iverson LL (1966) Regional studies of catecholamines in the rat brain. The deposition of [3H]norepinephrine, [3H]dopamine, and [3H]dopa in various regions of the brain. J Neurochem 13(8):655–669
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Wendel A (1981) Glutathione peroxidase. In: Jakoly WB (ed) Methods in enzymology, vol 7. Academic, New York, pp 325–333
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Kuruvilla A, Pillay VV, Venkatesh T et al (2004) Portable lead analyzer to locate source of lead. Indian J Pediat 71:495–499
Quinlan GJ, Halliwell B, Moorhouse CP, Gutteridge JMC (1988) Action of lead(II) and aluminium(III) ions on iron-stimulated lipid peroxidation in liposomes, erythrocytes and rat liver microsomal fractions. Biochim Biophys Acta 962:196–200
Zhao Y, Wang L, Shen HB, Wang ZX, Wei QY, Chen F (2007) Association between delta-aminolevulinic acid dehydratase (ALAD) polymorphism and blood lead levels: a meta-regression analysis. J Toxicol Environ Health A 70:1986–1994
Flora SJS, Flora G, Saxena G, Mishra M (2007) Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol 53:24–46
Gleichmann M, Mattson MP (2011) Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal 14(7):1261–1273
Hermes-Lima M, Pereira B, Bechara EJH (1991) Are free radicals involved in lead poisoning? Xenobiotica 21:1085–1090
Sandhir R, Julka D, Gill KD (1994) Lipoperoxidative damage on lead exposure in rat brain and its implications on membrane bound enzymes. Pharmacol Toxicol 74:66–71
Schwartz BS, Lee BK, Lee GS (2000) Associations of blood lead, dimercaptosuccinic acid-chelatable lead and tibia lead with polymorphisms in the vitamin D receptor and 5-aminolevulinic acid dehydratase genes. Environ Health Perspect 108:949–954
Shukla PK, Khanna VK, Khan MY, Srimal RC (2003) Protective effect of curcumin against lead neurotoxicity in rat. Hum Exp Toxicol 22(12):653–658
Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK et al (2006) Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J 20(6):788–790
Blaschke AJ, Weiner JA, Chun J (1998) Programmed cell death is a universal feature of embryonic and postnatal neuroproliferative regions throughout the central nervous system. J Comp Neurol 396:39–50
Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC (2000) Bax oligomerization is required for channel-forming activity in liposomes and trigger cytochrome c release from mitochondria. Biochem J 345:271–278
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Acknowledgments
The authors would like to thank the Manipal University, India, for providing the infrastructure facilities.
Funding
This research received no specific grant from any funding agency in the public, commercial or non-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkur, R.R., Bairy, L.K. Assessment of Oxidative Stress in Hippocampus, Cerebellum and Frontal Cortex in Rat Pups Exposed to Lead (Pb) During Specific Periods of Initial Brain Development. Biol Trace Elem Res 164, 212–218 (2015). https://doi.org/10.1007/s12011-014-0221-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0221-3