Abstract
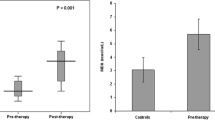
We aimed to investigate the effects of iron deficiency (ID) or iron-deficiency anemia (IDA) on oxidative stress and renal tubular functions before and after treatment of children. A total of 30 children with a diagnosis of IDA constituted the IDA group and 32 children with a diagnosis of ID constituted the ID group. Control group consisted 38 age-matched children. Serum ferritin, soluble transferrin receptor (sTfR), serum, and urinary sodium (Na), potassium (K), calcium (Ca), phosphorus (P), creatinine (Cr), uric acid (UA), urinary N-acetyl-β-d-glucosaminidase (NAG) levels, and intra-erythrocyte malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) levels were measured before and after iron therapy in the IDA and ID groups, whereas it was studied once in the control group. We have divided the study group in groups according to age (infants <2 years, children 3–9 years, and adolescents 10–15 years). Patients with IDA (infant, adolescent) and ID (infant, children, and adolescent) had a significantly high level of MDA in post-treatment period in comparison to those of healthy control. Patients with IDA (children, adolescent) and ID (infant, children) had a significantly high level of pre-treatment GSH-Px than controls. Post-treatment SOD was lower in IDA (children and adolescent) groups than control and post-treatment CAT was lower in IDA and ID (adolescent) groups than control. These findings show that ferrous sulfate used in the treatment of ID or IDA could lead to oxidative stress; however, a marked deterioration of in proximal renal tubular functions was not seen.
Similar content being viewed by others
References
Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19(2):164–174
Fenton HJH (1894) Oxidation of tartaric acid in the presence of iron. J Chem Soc 65:899–910
Lambeth JD, Neish AS (2014) Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol 9:119–145
Aust AE, Eveleigh JF (1999) Mechanisms of DNA oxidation. Proc Soc Exp Biol Med 222:246–252
Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Review Nat Chem Biol 10(1):9–17
Al-Ismaili Z, Piccioni M, Zappitelli M (2011) Rhabdomyolysis: pathogenesis of renal injury and management. Pediatr Nephrol 26:1781–1788
Fervenza FC, Croatt AJ, Bittar CM, Rosenthal DW, Lager DJ, Leung N, Zeldenrust SR, Nath KA (2008) Induction of heme oxygenase −1 and ferritin in the kidney in warm antibody hemolytic anemia. Am J Kidney Dis 52:972–977
Kaissling B, Spiess S, Rinne B, Le Hir M (1993) Effects of anemia on morphology of rat renal cortex. Am J Physiol 264:F608–F617
Özçay F, Derbent M, Aldemir D, Türkoğlu S, Baskın E, Özbek N, Saatçi Ü (2003) Effect of iron deficiency anemia on renal tubular function in childhood. Pediatr Nephrol 18:254–256
Price RG (1992) The role of NAG (N-acetyl-β-D-glucosaminidase) in the diagnosis of kidney disease including the monitoring of nephrotoxicity. Clin Nephrol 38:14–19
Kwatkowska E, Domański L, Bober J, Kłoda K, Safranow K, Szymańska-Pasternak J, Romanowski M, Sulecka A, Pawlik A, Ciechanowski K. (2014) N-acetyl-beta-glucosaminidase urine activity as a marker of early proximal tubule damage and a predictor of the long-term function of the transplanted kidneys. Acta Biochim Pol. Jun 11. [Epub ahead of print]
Kunin CM, Chesney RW, Craig WA, England AC, DeAngelis C (1978) Enzymuria as a marker of renal injury and disease: studies of N-acetyl-b-glucosaminidase in the general population and in patients with renal disease. Pediatrics 62:751–759
Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C; Centers for Disease Control and Prevention, National Center for Health Statistics. (2005) Hematological and iron-related analytes—reference data for persons aged 1 year and over: United States, 1988–94.Vital Health Stat 11. 247:1–156
Centers for Disease Control and Prevention (2002) Iron deficiency—United States, 1999–2000. MMWR Morb Mortal Wkly Rep 51:897–899
Avner ED, Harmon WE, Niaudet P, Yoshikawa N (2009) Pediatric nephrology. Springer-Verlag Berlin, Heidelberg
Geary DF, Schaefer F (2008) Comprehensive pediatric nephrology. Mosby Elsevier, Philadelphia
Elisaf M, Siamopoulos KC (1995) Fractional excretion of potassium in normal subject and in patient with hypokalemia. Postgrad Med J 71:211–212
Passwell JH, Modan M, Brish M, Orda S, Boichis H (1974) Fractional excretion of uric acid in infancy and childhood. Index of tubular maturation. Arch Dis Child 49(11):878–882
Pollak A, Hayde M, Hayn M, Herkner K, Lombard KA, Lubec G (2001) Effect of intravenous iron supplementation on erythropoiesis in erythropoietin-treated premature infant. Pediatrics 107:78–85
Dıaz-Castro J, Alferaz M, Lopez-Aliaga I, Nestares T, Granados S, Barrionuevo M, Campos MS (2008) Influence of nutritional iron deficiency anemia on DNA stability and lipid peroxidation in rats. Nutrition 24:1167–1173
Kumerova A, Lece A, Skesters A, Silova A, Petuhovs V (1998) Anemia and antioxidant defence of the red blood cells. Mater Med Pol 30:12–15
Aycicek A, Koc A, Oymak Y, Selek S, Kaya C, Guzel B (2014) Ferrous sulfate (Fe2+) had a faster effect than did ferric polymaltose (Fe3+) on increased oxidant status in children with iron-deficiency anemia. J Pediatr Hematol Oncol 36(1):57–61
Ray G, Husain SA (2002) Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol 40(11):1213–1232
McAnulty NS, Gropper SS, McAnulty SR, Keith R (2003) Iron depletion without anemia is not associated with impaired selenium status in college-aged women. Biol Trace Elem Res 91:125–136
Inal ME, Kanbak G, Sunal E (2001) Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta 305(1–2):75–80
Tekin D, Yavuzer S, Tekin M, Akar N, Cin Ş (2001) Possible effects of antioxidant status on increased platelet aggregation in childhood iron-deficiency anemia. Pediatr Int 43:74–77
Jansson LT, Perkkio M, Willis WT, Refino CJ, Dalman PR (1985) Red cell superoxide dismutase is increased in iron deficiency anemia. Acta Hematol 74:318–321
Isler M, Delibas N, Guclu M, Gultekin F, Sutcu R, Bahceci M, Kosar A (2002) Superoxide dismutase and glutathione peroxidase in erythrocytes of patients with iron deficiency anemia: effects of different treatment modalities. Croat Med J 43:16–19
Kurtoglu E, Ugur A, Baltaci AK, Undar L (2003) Effect of iron supplementation on oxidative stress and antioxidant status in iron-deficiency anemia. Biol Trace Elem Res 96:117–123
Kang DH (2002) Oxidative stress, DNA damage and breast cancer. AACN Clin Issues 13:540–549
Diplock AT, Rice-Evans AC, Burton RH (1994) Is there a significant role for lipid peroxidation in the causation of malignancy and for antioxidants in cancer prevention? Cancer Res 54:1952–1956
Seymen O, Seven A, Candan G, Yiğit G, Hatemi S, Hameti H (1997) The effect of iron supplementation on GSH levels, GSH-Px, and SOD activities of erythrocytes in L-thyroxine administration. Acta Med Okayama 51:129–133
Srigiridhar K, Nair KM (1998) Iron-deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Free Radic Biol Med 25(6):660–665
Díaz-Castro J, García Y, López-Aliaga I, Alférez MJ, Hijano S, Ramos A, Campos MS (2013) Influence of several sources and amounts of iron on DNA, lipid and protein oxidative damage during anaemia recovery. Biol Trace Elem Res 155(3):403–410
Huang J, Sun J, Li WX, Wang LJ, Wang AX, Huo JS, Chen JS, Chen CM (2009) Efficacy of different iron fortificants in wheat flour in controlling iron deficiency. Biomed Environ Sci 22(2):118–121
Mehta BC, Patel KB, Mehta JB (1989) Effect of iron deficiency on renal function. J Assoc Physicians India 37:685–686
Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW (2013) Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol 9(7):385–398
Smith CP, Thévenod F (2009) Iron transport and the kidney. Biochim Biophys Acta 1790:724–730
Authors’ Contributions
Demet Altun and Ahmet Emin Kurekci were the principal investigators and take primary responsibility for the paper. Orhan Gursel and Duygu Övunc Hacıhamdioglu recruited the patients. Ismail Kurt and Ahmet Aydin performed the laboratory work for this study. Ahmet Emin Kurekci and Okan Ozcan co-ordinated the research. Demet Altun and Orhan Gursel wrote the paper.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altun, D., Kurekci, A.E., Gursel, O. et al. Malondialdehyde, Antioxidant Enzymes, and Renal Tubular Functions in Children with Iron Deficiency or Iron-Deficiency Anemia. Biol Trace Elem Res 161, 48–56 (2014). https://doi.org/10.1007/s12011-014-0084-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0084-7