Abstract
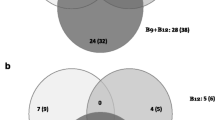
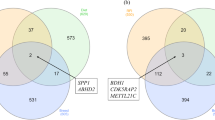
Selenium (Se) is an important trace mineral that, due to deficiencies in the soil in many parts of the USA, must be supplemented directly to the diet of foraging cattle. Both organic and inorganic forms of dietary Se supplements are available and commonly used, and it is known that Se form affects tissue assimilation, bioavailability, and physiological responses. However, little is known about the effects of form of dietary Se supplements on gene expression profiles, which ostensibly account for Se form-dependent physiological processes. To determine if hepatic transcriptomes of growing beef (Angus-cross) heifers (0.5 kg gain/day) was altered by form of dietary supplemental Se, none (Control), or 3 mg Se/day as inorganic Se (ISe, sodium selenite), organic (OSe, Sel-Plex®), or a blend of ISe and OSe (1.5 mg:1.5 mg, Mix) Se was fed for 168 days, and the RNA expression profiles from biopsied liver tissues was compared by microarray analysis. The relative abundance of 139 RNA transcripts was affected by Se treatment, with 86 of these with complete gene annotations. Statistical and bioinformatic analysis of the annotated RNA transcripts revealed clear differences among the four Se treatment groups in their hepatic expression profiles, including (1) solely and commonly affected transcripts; (2) Control and OSe profiles being more similar than Mix and ISe treatments; (3) distinct OSe-, Mix-, and ISe-Se treatment-induced “phenotypes” that possessed both common and unique predicted physiological capacities; and (4) expression of three microRNAs were uniquely sensitive to OSe, ISe, or Mix treatments, including increased capacity for redox potential induced by OSe and Mix Se treatments resulting from decreased expression of MiR2300b messenger RNA. These findings indicate that the form of supplemental dietary Se consumed by cattle will affect the composition of liver transcriptomes resulting, presumably, in different physiological capacities.



Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BOLA-DQA2:
-
Bos taurus major histocompatibility complex, class II, DQ alpha 2
- DEG:
-
Differentially expressed gene
- DDHD1:
-
Phosphatidic acid-prefering phospholipase A1
- GHRH:
-
Growth hormone-releasing hormone
- GPX1:
-
Cellular glutathione peroxidase-1
- ISe:
-
Inorganic selenium
- miRNA:
-
microRNA
- mRNA:
-
Messenger RNA
- Mix:
-
50:50 ISe:OSe
- OSe:
-
Organic selenium
- QPCT:
-
Glutaminyl-peptide cyclotransferease
- Se:
-
Selenium
- SeCys:
-
Selenocysteine
- SeMet:
-
Selenomethionine
- SEPW:
-
Selenoprotein W
References
Suzuki KT (2005) Metabolomics of selenium: Se metabolites based on speciation studies. J Health Sci 51:107–114
Pehrson B (1993) Diseases and diffuse disorders related to selenium deficiencies in ruminants. Nor J Agric Sci 11:79–93
Sordillo LM, Aitken SL (2009) Impact of oxidative stress on the health and immune function of dairy cattle. Vet Immunol Immunopathol 128:104–109
National Research Council (1996) Minerals. In: Nutrient requirements of beef cattle, 7th revised edition. 2001 Update. National Academy Press, Washington, DC, pp 54–74
Ammerman CB, Miller SM (1975) Selenium in ruminant nutrition: a review. J Dairy Sci 58:1561–1571
Dargatz DA, Ross PF (1996) Blood selenium concentrations in cows and heifers on 253 cow-calf operations in 18 states. J Anim Sci 74:2891–2895
Food and Drug Administration (1987) Food additives permitted in feed and drinking water of animals: selenium. Fed Reg 52:10668
Korhola M, Vainio A, Edelmann K (1986) Selenium yeast. Ann Clin Res 18:65–68
Liao SF, Brown KR, Stromberg AJ, Burris WR, Boling JA, Matthews JC (2011) Dietary supplementation of selenium in inorganic and organic forms differentially and commonly alters blood and liver selenium concentrations and liver gene expression profiles of growing beef heifers. Biol Trace Elem Res 140:151–169
Brennan KM, Burris WR, Boling JA, Matthews JC (2011) Selenium content in blood fractions and live of beef heifers is greater with a mix of inorganic/organic or organic versus inorganic supplemental selenium but the time required for maximal assimilation is tissue-specific. Biol Trace Elem Res 144:504–516
Ortman K, Pehrson B (1999) Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J Anim Sci 77:3365–3370
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Wu Z, Irizarry RA, Gentleman R, Murillo FM, Spencer F (2004) A model based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc 99:909–917
Partek D (2009) Partek documentation: turning data into discovery. Partek Incorporated, St. Louis
Lewis BP, Burge CP, Bartel DP (2005) Conserved seed pairing, oftern flanked by adenosine, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalians are conserved targets of microRNAs. Genome Res 19:92–105
Fischer A, Pallauf J, Gohil K, Weber SU, Packer L, Rimbach G (2001) Effect of selenium and vitamin E deficiency on differential gene expression in rat liver. Biochem Biophys Res Commun 285:470–475
Huang J-Q, Li D-L, Zhao H, Sun L-H, Xia X-J, Wang K-N, Luo X, Lei XG (2011) The selenium deficiency disease exudative diathesis in chicks is associated with down regulation of seven common selenoprotein genes in liver and muscle. J Nutr 141:1605–1610
Sun B, Wang RH, Li J, Jiang Z, Xu S (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143:1516–1523
Ullrey DE (1987) Biochemical and physiological indicators of selenium status in animals. J Anim Sci 65:1712–1726
Corah LR (1996) Trace mineral requirements of grazing cattle. Anim Feed Sci Technol 59:61–70
Surai PF (2006) Selenium in ruminant nutrition. In: Selenium in nutrition and health. Nottingham University Press, Nottingham, UK, pp 487–587
Jeong D, Kim TS, Chung YW, Lee BJ, Kim IY (2002) Selenoprotein W is a glutathione-dependent antioxidant in vivo. FEBS Lett 517:225–228
Mori Y, Kajimoto T, Nakao A, Takahashi N, Kiyonaka S (2011) Receptor signaling integration by TRP channelsomes. Adv Exp Med Biol 704:373–389
Yamamoto S, Takahashi N, Mori Y (2010) Chemical physiology of oxidative stress-activated TRPM2 and TRPC5 channels. Prog Biophys Mol Biol 103:18–27
Bon RS, Beech DJ (2013) In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels – mirage or pot of gold? Br J Pharmacol 170:459–474
Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN (2002) mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res 109:95–104
Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ (2008) TRPC channel activation by extracellular thioredoxin. Nature 451:69–72
Rychkov GY, Barritt G (2011) Expression and function of TRP channels in liver cells. Adv Exp Med Biol 704:667–686
Guo H, Nairn A, dela Rosa M, Nagy T, Zhao S, Moreman K, Pierce M (2012) Transcriptional regulation of the protocadherin β lucster during Her-2 protein-induced mammary tumorigenesis results from altered N-glycan branching. J Biol Chem 287:24941–24954
Kitagishi Y, Matsuda S (2013) RUFY, Rab and Rap family proteins involved in a regulation of cell polarity and membrane trafficking. Int J Mol Sci 14:6487–6498
Nakazawa H, Sada T, Toriyama M, Tago K, Sugiura T, Fukuda M, Inagaki N (2012) Rab33a mediates anterograde vesicular transport for membrane exocytosis and axon outgrowth. J Neurosci 32:12712–12725
Tsuneoka Y, Matsuo Y, Higuchi R, Ichikawa Y (1992) Characterization of the cytochrome P-450IID subfamily in bovine liver. Nucleotide sequences and microheterogeneity. Eur J Biochem 280:739–746
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–111
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogen J 5:6–13
Kimura T, Sakisaka T, Baba T, Yamada T, Takai Y (2006) Involvement of the Ras-Ras-activated Rab5 guanine nucleotide exchange factor RIN2-Rab5 pathway in the hepatocyte growth factor-induced endocytosis of E-cadherin. J Biol Chem 281:10598–10609
Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, Del Conte-Zerial P, Hengstler JG, Kalaidzidis Y, Koteliansky V, Zerial M (2012) Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature 485:465–470
Yamashita A, Oka S, Tanikawa T, Hayashi Y, Neomoto-Sasaki Y, Sugiura T (2013) The actions and metabolism of lysophosphatidylinositol, an endogenous agonist for GPR55. Prostagland Lipid Mediat 107:103–116
Higgs HN, Han MH, Johnson GE, Glomset JA (1998) Cloning of a phosphatidic acid-preferring phospholipase A1 from bovine testis. J Biol Chem 273:5468–5477
Moreno-Navarrette JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar F, Guzmán R, Gómez-Ambrosi J, Pulido MR, Russell WR, Imbernón M, Ross RA, Malagón MM, Dieguez C, Fernández-Real JM, Frühbeck G, Nogueiras R (2012) The l-alpha-lysophosphatidylinositol/GPR55 system and its potential role in obesity. Diabetes 61:281–291
Simocks AC, O’Keefe L, Jenkin KA, Mathai ML, Hryciw DH, McAinch AJ (2011) A potential role for GPR55 in the regulation of energy homeostasis. Drug Discov Today. doi:10.1016/j.drudis.2013.12.005
Svitkin YV, Pause A, Haghighat A, Pyronnet S, Witherell G, Belsham GJ, Sonenberg N (2001) The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA 7:382–394
Wang FW, Ulyanova NP, van der Waal MS, Patnaik D, Lens SM, Higgins JM (2011) A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol 21:1061–1069
Okado H, Ohataka-Maruyama C, Sugitani Y, Fukuda Y, Ishida R, Hirai S, Miwa A, Takahashi A, Aoki K, Mochida K, Suzuki O, Honda T, Nakajima K, Ogawa M, Terashima T, Matsuda J, Kawano H, Kasai M (2009) The transcriptional repressor RP58 is crucial for cell-division patterning and neuroanl survival in the developing cortex. Dev Biol 331:140–151
Kelly KF, Daniel JM (2006) POZ for effect-POZ-ZF transcription factors in cancer and development. Trends Cell Biol 16:578–587
Fischer WH, Spiess J (1987) Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. PNAS USA 84:3628–3632
Cynis H, Kehlen A, Haegele M, Hoffmann T, Fujii U, Shibazaki Y, Yoneyama H, Schilling S, Demuth HU (2013) Inhibition of glutaminyl cyclases alleviates CCL2-mediated inflammation of non-alcoholic fatty liver disease in mice. Int J Exp Pathol 94:217–225
Friedman SL (2000) Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275:2247–2250
Yakubenko VP, Bhattacharjee A, Pluskota E, Cathcart MK (2011) alphaMbeta2 Integrin activation prevents alternative activation of human and murine macrophages and impedes foam cell formation. Circ Res 108:544–554
Zaia KA, Reimer RJ (2009) Synaptic vesicle protein NTT4/XT1 (SLC6A17) catalyzes Na+-coupled neutral amino acid transport. J Biol Chem 284:8439–8448
Hohla F, Moder A, Mayrhauser U, Hauser-Kronberger C, Schally AV, Varga JL, Zarandi M, Buchholz S, Huber R, Aigner E, Ritter M, Datz C (2008) Differential expression of GHRH receptor and its splice variant 1 in human normal and malignant mucosa of the oesophagus and colon. Int J Oncol 33:137–143
Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV (2011) Growth hormone-releasing hormone: not only a neurohormone. Trends Endocrinol Metab 22:311–317
Christodoulou C, Schally AV, Chatzistamou I, Kondi-Pafiti A, Lamnissou K, Kouloheri S, Kalofoutis A, Kiaris H (2006) Expression of growth hormone-releasing hormone (GHRH) and splice variant of GHRH receptors in normal mouse tissues. Regul Pept 136:105–108
Bagnato A, Moretti C, Ohnishi J, Frajese G, Catt KJ (1992) Expression of the growth hormone-releasing hormone gene and its peptide product in the rat ovary. Endocrinology 130:1097–1102
Berry SA, Srivastava CH, Rubin LR, Phipps WR, Pescovitz OH (1992) Growth hormone-releasing hormone-like messenger ribonucleic acid and immunoreactive peptide are present in human testis and placenta. J Clin Endocrinol Metab 75:281–284
Bosman FT, Van Assche C, Nieuwenhuyzen Kruseman AC, Jackson S, Lowry PJ (1984) Growth hormone-releasing factor (GRF) immunoreactivity in human and rat gastrointestinal tract and pancreas. J Histochem Cytochem 32:1139–1144
Sesma JI, Esther CR Jr, Kreda SM, Jones L, O’Neal W, Nishihara S, Nicholas RA, Lazarowski ER (2009) Endoplasmic reticulum/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 284:12572–12583
Ishida N, Kuba T, Aoki K, Miyatake S, Kawakita M, Sanai Y (2005) Identification and characterization of human Golgi nucleotide sugar transporter SLC35D2, a novel member of the SLC35 nucleotide sugar transporter family. Genomics 85:106–116
Xu J, Morinaga H, Oh D, Li P, Chen A, Talukdar S, Mamane Y, Mancini JA, Nawrocki AR, Lazarowski E, Olefsky JM, Kim JJ (2012) GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity. J Immunol 189:1992–1999
Zhang G, Hao C, Lou Y, Xi W, Wang X, Wang Y, Qu Z, Guo C, Chen Y, Zhang Y, Liu S (2010) Tissue-specific expression of TIPE2 provides insights into its function. Mol Immunol 47:2435–2442
Zhang L, Shi Y, Wang Y, Zhu F, Wang Q, Ma C, Chen YH, Zhang L (2011) The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol 48:1209–1215
Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X, Chen YH (2008) TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133:415–426
Lou Y, Liu S, Zhang C, Zhang G, Li J, Ni M, An G, Dong M, Liu X, Zhu F, Zhang W, Gao F, Chen YH, Zhang Y (2013) Enhanced atherosclerosis in TIPE-2deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol 191:4849–4857
Chaudhary K, Liedtke C, Wertenbruch S, Trautwein C, Streetz KL (2013) Caspase8differentially controls hepatocytes and non-parenchymal liver cells during chronic cholestatic liver injury in mice. J Hepatol 59:1292–1298
Gus-Brautbar Y, Johnson D, Zhang L, Sun H, Wang P, Zhang S, Zhang L, Chen YH (2012) The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol Cell 45:610–618
Zeng Q, Subramaniam VN, Wong SH, Tang BL, Parton RG, Rea S, James DE, Hong W (1998) A novel synaptobrevin/VAMP homologous protein (VAMP5) is increased during in vitro myogenesis and present in the plasma membrane. Mol Biol Cell 9:2423–2437
Hasan N, Corbin D, Hu C (2010) Fusogenic pairings of vesicle-associated membrane proteins (VAMPs) and plasma membrane t-SNAREs–VAMP5 as the exception. PLoS ONE 5:e14238
Ko J, Fuccillo MV, Malenka RC, Südh (2009) LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron 64:791–798
Bang ML, Owczarek S (2013) A matter of balance: role of neurexin and neuroligin at the synapse. Neurochem Res 38:1174–1189
Laurén J, Airaksinen MS, Saarma M (2003) Timmusk T (2003) A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics 81:411–421
Soler-Llavina GJ, Fuccillo MV, Ko J, Sudhof TC, Malenka RC (2011) The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. PNAS 108:16502–16509
Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123:3685–3692
Morooka A, Asahina M, Kohda C, Tajima S, Niimi M, Nishino Y, Sugiyama M, Aida Y (1995) Nucleotide sequence and the molecular evolution of a new A2 gene in the DQ subregion of the bovine major histocompatibility complex. Biochem Biophys Res Commun 212:110–117
Handunnetthi L, Ramagopalan SV, Ebers GC, Knight JC (2009) Regulation of MHC II gene expression, genetic variation, and disease. Genes Immun 11:99–112
Hou Q, Huang J, Ju Z, Li Q, Li L, Wang C, Sun T, Wang L, Hou M, Hang S, Zhong J (2012) Identification of splice variants, targeted microRNAs and functional single nucleotide polymorphisms of the BOLA-DQ2 gene in dairy cattle. DNA Cell Biol 31:739–744
Zimin AV, Delcher AL, Florea L, Kelley DR, Schatz MC, Puiu D, Hanrahan F, Pertea G, Van Tassell CP, Sonstegard TS, Marçais G, Roberts M, Subramanian P, Yorke JA, Salzberg SL (2009) A whole genome-assembly of the domestic cow, Bos taurus. Genome Biol 10(4):R42
Ding W, Fujimura M, Mori A, Tooyama I, Kimura H (1991) Light and electron microscopy of neuropeptide Y-containing nerves in human liver, gallbladder, and pancreas. Gastroenterology 101:1054–1059
El-Salhy M (2000) Neuropeptide levels in murine liver and biliary pathways. Ups J Med Sci 105:207–213
Kim HY, Cho S, Yu J, Sung S, Kim H (2010) Analysis of copy number variation in 8,842 Korean individuals reveals 39 genes associated with hepatic biomarkers AST and ALT. BMB Rep 43:547–553
Yamashita A, Kumazawa T, Koga H, Suzuki N, Oka S, Sugiura T (2010) Generation of lysophosphatidylinositol by DDHD domain containing 1 (DDHD1): possible involvement of phospholipase D/phosphatidic acid in the activation of DDHD1. Biochim Biophys Acta 1801:711–720
Savulescu AF, Glickman MH (2011) Proteasome activator 200: The HEAT is on…. Mol Cell. doi:10.1074/mcp.M110.006890
Ustrell V, Hoffman L, Pratt G, Rechsteiner M (2002) PA200, a nuclear proteasome activator involved in DNA repair. EMBO 21:3516–3525
Khor B, Bredemeyer AL, Huang CY, Turnbull IR, Evans R, Maggi LB Jr, White JM, Walker LM, Carnes K, Hess RA, Sleckman BP (2006) Proteasome activator PA200 is required for normal spermatogenesis. Mol Cell Biol 26:2999–3007
Davies KJ (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83:301–310
Jung T, Bader N, Grune T (2007) Oxidized proteins: intracellular distribution and recognition by the proteasome. Arch Biochem Biophys 462:231–237
Gong D, Ferrell JE Jr (2010) The roles of cyclin A2, B1, and B2 in early and late mitotic events. Mol Biol Cell 21:3149–3161
Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH (2001) Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci U S A 98:11468–11473
Alves LA, Coutinho-Silva R, Persechini PM, Spay DC, Savino W, Campos de Carvalho AC (1996) Are there functional gap junctions or junctional hemichannels in macrophages? Blood 88:328–334
Alves LA, Campos de Carvalho AC, Savino W (1998) Gap junctions: a novel route for direct cell-cell communication in the immune system. Immunol Today 19:269–275
Ilardi JM, Mochida S, Sheng ZH (1999) Snapin: a SNARE-associated protein implicated in synaptic transmission. Nat Neurosci 2:119–124
Pan PY, Tian JH, Sheng ZH (2009) Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron 61:412–424
Acknowledgments
This research was supported by the Alltech-University of Kentucky Animal Nutrigenomics Alliance (JCM), University of Kentucky, and Kentucky Agricultural Experiment Station. The information reported in this paper (publication no. 14-07-038) is part of a project of the Kentucky Agricultural Experiment Station and is published with approval of the Director.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matthews, J.C., Zhang, Z., Patterson, J.D. et al. Hepatic Transcriptome Profiles Differ Among Maturing Beef Heifers Supplemented with Inorganic, Organic, or Mixed (50 % Inorganic:50 % Organic) Forms of Dietary Selenium. Biol Trace Elem Res 160, 321–339 (2014). https://doi.org/10.1007/s12011-014-0050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0050-4