Abstract
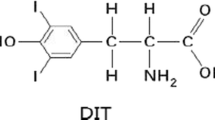
Iodine excess is emerging as a new focus. A better understanding of its hazardous effects on the liver will be of great benefit to health. The aim of this study is to illustrate the effects of iodine excess on hepatic lipid homeostasis and explore its possible mechanisms. One hundred twenty BaLB/c mice were given iodine at different levels (0, 0.3, 0.6, 1.2, 2.4, and 4.8 mg I/L) in drinking water for 1 or 3 months. Lipid parameters and serum thyroid hormones were measured. Hepatic type 1 deiodinase activity and oxidative stress parameters were evaluated. The mRNA expression of sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) was detected by real-time polymerase chain reaction. Dose-dependent increase of hepatic triglyceride content was detected (r = 0.680, P < 0.01) in iodine-loaded groups. Evident hepatic steatosis was observed in 2.4 and 4.8 mg I/L iodine-loaded groups. The activities of antioxidant enzymes (glutathione peroxidase and superoxide dismutase) were decreased, and the malondialdehyde level was increased by excessive iodine in both serum and liver in a dose-dependent manner, accompanying the decrease of hepatic D1 activity. That resulted in the increase of serum total thyroxine and the decrease of serum total triiodothyronine in iodine-loaded groups. The mRNA expression of SREBP-1c and FAS was increased in iodine-loaded groups in response to the change of serum triiodothyronine. Present findings demonstrated that iodine excess could dose dependently induce hepatic steatosis. Furthermore, our data suggested that the disturbance of thyroid hormone metabolism involving oxidative stress may play a critical role in iodine excess-induced hepatic steatosis.




Similar content being viewed by others
References
Delange F, Lecomte P (2000) Iodine supplementation: benefits outweigh risks. Drug Saf 22:89–95
Guo H, Yang X et al (2006) Effect of selenium on thyroid hormone metabolism in filial cerebrum of mice with excessive iodine exposure. Biol Trace Elem Res 113:281–295
Rose NR, Bonita R et al (2002) Iodine: an environmental trigger of thyroiditis. Autoimmun Rev 1:97–103
Roti E, Uberti ED (2001) Iodine excess and hyperthyroidism. Thyroid 11:493–500
Yang XF, Xu J et al (2006) Developmental toxic effects of chronic exposure to high doses of iodine in the mouse. Reprod Toxicol 22:725–730
Kroupova VKP, Kaufmann S et al (1998) Metabolic effects of giving additional iodine to laying hens. Vet Med (Praha) 7:207–212
Perry GC, Lewis PD et al (1989) Iodine supplementation from two sources and its effect on egg output. Br Poult Sci 30:973–974
Perry GC, Lewis PD et al (1990) Responses of the laying hen to dietary iodine supplementation. Proceedings of the 8th European poultry conference. Barcelona: Spanish Branch. World's Poult Sci Assoc 1:384–387
Bolt MW, Card JW et al (2001) Disruption of mitochondrial function and cellular ATP levels by amiodarone and N-desethylamiodarone in initiation of amiodarone-induced pulmonary cytotoxicity. J Pharmacol Exp Ther 298:1280–1289
Card JW, Lalonde BR et al (1998) Amiodarone-induced disruption of hamster lung and liver mitochondrial function: lack of association with thiobarbituric acid-reactive substance production. Toxicol Lett 98:41–50
Fromenty B, Fisch C et al (1990) Amiodarone inhibits the mitochondrial beta-oxidation of fatty acids and produces microvesicular steatosis of the liver in mice. J Pharmacol Exp Ther 255:1371–1376
Koenig RJ (2005) Regulation of type 1 iodothyronine deiodinase in health and disease. Thyroid 15:835–840
Maiti PK, Kar A (1997) Dimethoate inhibits extrathyroidal 5′-monodeiodination of thyroxine to 3,3′,5-triiodothyronine in mice: the possible involvement of the lipid peroxidative process. Toxicol Lett 14:1–6
Brzezińska-Slebodzińska E (2001) Fever induced oxidative stress: the effect on thyroid status and the 5′-monodeiodinase activity, protective role of selenium and vitamin E. J Physiol Pharmacol 52:275–284
Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K et al (1999) Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem 274:35832–35839
Ahmed MH, Byrne CD (2007) Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug Discov Today 12:740–747
Viguerie N, Langin D (2003) Effect of thyroid hormone on gene expression. Curr Opin Clin Nutr Metab Care 6:377–381
Viguerie N, Millet L et al (2002) Regulation of human adipocyte gene expression by thyroid hormone. J Clin Endocrinol Metab 87:630–634
Kawai K, Sasaki S et al (2004) Unliganded thyroid hormone receptor-1 represses liver X receptor/oxysterol-dependent transactivation. Endocrinology 145:5515–5524
Hashimoto K, Yamada M et al (2006) Mouse sterol response element binding protein-1c gene expression is negatively regulated by thyroid hormone. Endocrinology 147:4292–4302
Chinese Nutrition Society (2001) Chinese dietary reference intakes, DRIs. Acta Nntrimenta Sinica 23:193-196
Gao Q, Zhang S, Xu C et al (2002) The dose–response relationship study between the quantitative morphological stereology on thyroid and different iodine doses in mice. Zhonghua Yu Fang Yi Xue Za Zhi 36:38–40
Gao B, Yin G (1997) Effects of high-dose iodine on brain development in mice. Zhonghua Yu Fang Yi Xue Za Zhi 31:134–136
Zhao LN, Xu J, Peng XL et al (2010) Dose and time-dependent hypercholesterolemic effects of iodine excess via TRb1-mediated down regulation of hepatic LDLr gene expression. Eur J Nutr 49:257–265
Fischer PW, L'Abbe MR et al (1986) Colorimetric determination of total iodine in foods by iodide-catalyzed reduction of Ce+4. J Assoc Off Anal Chem 69:687–689
Folch J, Lees M et al (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Benzie IF, Strain JJ (1974) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Hafeman DG, Sunde RA et al (1974) Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr 104:580–587
Winterbourn CC, Hawkins RE et al (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85:337–341
Placer ZACL, Johnson BC et al (1966) Estimation of product of lipid peroxidation, malondialdehyde in biochemical. system. Anal Biochem 16:359–367
Koopdonk-Kool JM, de Vijlder JJ et al (1996) Type II and type III deiodinase activity in human placenta as a function of gestational age. J Clin Endocrinol Metab 81:2154–2158
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C (T)) method. Methods 25:402–408
Santangeli P, Di Biase L et al (2012) Examining the safety of amiodarone. Expert Opin Drug Saf 11:191–214
Padmanabhan H (2010) Amiodarone and thyroid dysfunction. South Med J 103:922–930
Song M, Kim YJ et al (2011) Phospholipidosis induced by PPARγ signaling in human bronchial epithelial (BEAS-2B) cells exposed to amiodarone. Toxicol Sci 120:98–108
Hudig F, Bakker O et al (1998) Amiodarone decreases gene expression of low-density lipoprotein receptor at both the mRNA and the protein level. Metabolism 47:1052–1057
Rizzo LF et al (2012) Amiodarone and thyroid dysfunction. Article Span 72:63–74
Davis RA (1999) Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochim Biophys Acta 1440:1–31
Joanta AE, Filip A, Daicoviciu D (2006) Iodide excess exerts oxidative stress in some target tissues of the thyroid hormones. Acta Physiol Hung 93:347–359
Vitale M, Di Matola T et al (2000) Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology 141:598–605
Acknowledgments
This work was supported by the National Natural Science Foundation of China, no. 81102127 and 81172668. There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, Y., Qu, W., Zhao, LN. et al. Iodine Excess Induces Hepatic Steatosis Through Disturbance of Thyroid Hormone Metabolism Involving Oxidative Stress in BaLB/c Mice. Biol Trace Elem Res 154, 103–110 (2013). https://doi.org/10.1007/s12011-013-9705-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9705-9