Abstract
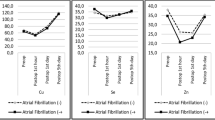
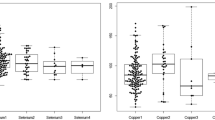
Trace elements may contribute to myocardial dysfunction and susceptibility of the phospholipid cell membrane to free-radical damage and oxidative changes. We studied the concentration of trace elements copper, zinc, and magnesium in cardiac surgery. Fifty-four consecutive patients for elective coronary artery bypass grafting (n = 30) and valve replacement (n = 24) were studied. Blood samples were collected every 30 min (T1–T5) during cardiopulmonary bypass and postoperatively (T6–T9). Plasma concentrations of copper, zinc, and magnesium were measured with flame atomic absorption spectrophotometry. The concentrations of copper, zinc, and magnesium were significantly different during and after cardiopulmonary bypass (p < 0.01). The zinc concentration at T7 and T8 (p < 0.01) and the copper concentration at T1, T9 (p < 0.05) were significantly different between two groups. However, the magnesium concentration had no significant differences between the two groups (p > 0.05). In patients undergoing valve replacement or coronary artery bypass grafting, the concentrations of copper and zinc decreased significantly during cardiopulmonary bypass. Our study suggests that the current cardiopulmonary bypass protocol is adequate in the maintenance of c magnesium. However, the low copper and zinc concentrations found in the present study may suggest that in the future, supplementation particularly of copper and zinc may become a necessary procedure in cardiac surgery with cardiopulmonary bypass.



Similar content being viewed by others
References
Clermont G, Vergely C, Jazayeri S, Lahet JJ, Goudeau JJ, Lecour S, David M, Rochette L, Girard C (2002) Systemic free radical activation is a major event involved in myocardial oxidative stress related to cardiopulmonary bypass. Anesthesiology 96:80–87. doi:10.1097/00000542-200201000-00019
Christen S, Finckh B, Lykkesfeldt J, Gessler P, Frese-Schaper M, Nielsen P, Schmid ER, Schmitt B (2005) Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med 38:1323–1332. doi:10.1016/j.freeradbiomed.2005.01.016
Bolli R, Jeroudi MO, Patel BS, Aruoma OI, Halliwell B, Lai EK, McCay PB (1989) Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion: evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ Res 65:607–622. doi:10.1161/01.RES.65.3.607
Valko M, Morris H, Cronin MT (2005) Metals toxicity and oxidative stress. Curr Med Chem 12:1161–1208. doi:10.2174/0929867053764635
Bray TM, Bettger WJ (1990) The physiological role of zinc as an antioxidant. Free Radic Biol Med 8:281–291. doi:10.1016/0891-5849(90)90076-U
Powell SR (2000) The antioxidant properties of zinc. J Nutr 130(Suppl 5S):S1447–S1454
Hennig B, Toborek M, Mcclain CJ (1996) Antiatherogenic properties of zinc: implications in endothelial cell metabolism. Nutrition 12:711–717. doi:10.1016/S0899-9007(96)00125-6
de Lorgeril M, Salen P, Accominotti M, Cadau M, Steghens JP, Boucher F, de Leiris J (2001) Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. Eur J Heart Fail 3:661–669. doi:10.1016/S1388-9842(01)00179-9
Shokrzadeh M, Ghaemian A, Salehifar E, Aliakbari S, Saravi SS, Ebrahimi P (2009) Serum zinc and copper levels in ischemic cardiomyopathy. Biol Trace Elem Res 127:116–123. doi:10.1007/s12011-008-8237-1
Medeiros DM, Liao Z, Hamlin RL (1992) Electrocardiographic activity and cardiac function in copperrestricted rats. Proc Soc Exp Biol Med 200:78–84. doi:10.3181/00379727-200-43396
Tang YR, Zhang SQ, Xiong Y, Zhao Y, Fu H, Zhang HP, Xiong KM (2003) Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol Trace Elem Res 92:97–104. doi:10.1385/BTER:92:2:97
Elin RJ (1994) Magnesium: the fifth but forgotten electrolyte. Am J Clin Pathol 102:616–622
Altura BM, Altura BT (1996) Role of magnesium in pathophysiological processes and the clinical utility of magnesium ion selective electrodes. Scand J Clin Lab Invest Suppl 56:211–234. doi:10.3109/00365519609088642
Taggart DP, Fraser WD, Shenkin A, Wheatley DJ, Fell GS (1990) The effects of intraoperative hypothermia and cardiopulmonary bypass on trace metals and their protein binding ratios. Eur J Cardiothorac Surg 4:587–594. doi:10.1016/1010-7940(90)90017-T
Zamparelli R, Carelli G, Pennisi MA, Baruffi E, Schiavello R, Intonti MA, Intonti F (1986) Zinc and copper metabolism during open-heart surgery. Scand J Thorac Cardiovasc Surg 20:241–245. doi:10.3109/14017438609105932
Dementéva II, Andrianova MI, Dzemeshkevich SL, Iavorovskiĭ AG, Lokshin LS (1993) Changes in the content of microelements—copper, zinc and iron—in the blood of patients following cardiopulmonary bypass. Anesteziol Reanimatol 4:50–53
Nuutinen LS, Ryhänen P, Pihlajaniemi R, Hollmén A, Tyrväinen L (1981) The levels of zinc, copper, calcium and magnesium in serum and urine after heart-valve replacement. Effects of oxygenator type and postoperative parenteral nutrition. Infusionsther Klin Ernahr 8:214–217. doi:10.1159/000221220
Chvapil M (1976) Effect of zinc on cells and biomembranes. Med Clin North Am 60:799–812
Riordan JF (1976) Biochemistry of zinc. Med Clin North Am 60:661–674
Zamparelli R, Carelli G, Pennisi MA, Baruffi E, Schiavello R, Intonti MA, Intonti F (1986) Zinc and copper metabolism during open-heart surgery. Scand J Thor Cardiovasc Surg 20:241–245. doi:10.3109/14017438609105932
Ghaemian A, Salehifar E, Jalalian R, Ghasemi F, Azizi S, Masoumi S, Shiraj H, Mohammadpour RA, Bagheri GA (2011) Zinc and copper levels in severe heart failure and the effects of atrial fibrillation on the zinc and copper status. Biol Trace Elem Res 143:1239–1246. doi:10.1007/s12011-011-8956-6
Kienast A, Roth B, Bossier C, Hojabri C, Hoeger PH (2007) Zinc-deficiency dermatitis in breast-fed infants. Eur J Pediatr 166:189–194. doi:10.1007/s00431-006-0218-9
Ackland ML, Michalczyk A (2006) Zinc deficiency and its inherited disorders—a review. Genes Nutr 1:41–49. doi:10.1007/BF02829935
Myung SJ, Yang SK, Jung HY, Jung SA, Kang GH, Ha HK, Hong WS, Min YI (1998) Zinc deficiency manifested by dermatitis and visual dysfunction in a patient with Crohn’s disease. J Gastroenterol 33:876–879. doi:10.1007/s005350050192
Chipperfield B, Chipperfield JR (1978) Differences in metal content of the heart muscle in death from ischemic heart disease. Am Heart J 95:732–737. doi:10.1016/0002-8703(78)90503-3
Chvapil M, Ryan JN, Zukoski CF (1972) Effects of zinc on lipid peroxidation in liver microsomes and mitochondria. Proc SOC Exp Biol Med 141:150–153. doi:10.3181/00379727-141-36734
O’Dell BL (1976) Biochemistry of copper. Med Clin North Am 60:687–703
Tang YR, Zhang SQ, Xiong Y, Zhao Y, Fu H, Zhang HP, Xiong KM (2003) Studies of five microelement contents in human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol Trace Elem Res 92:97–104. doi:10.1385/BTER:92:2:97
Milne DB, Davis CD, Nielsen FH (2001) Low dietary zinc alters indices of copper function and status in postmenopausal women. Nutrition 17:701–708. doi:10.1016/S0899-9007(01)00560-3
Cousins RJ (1985) Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev 65:238–309
Ungvári Z, Gupte SA, Recchia FA, Bátkai S, Pacher P (2005) Role of oxidative–nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol 3:221–229. doi:10.2174/1570161054368607
Wykretowicz A, Furmaniuk J, Smielecki J, Deskur-Smielecka E, Szczepanik A, Banaszak A, Wysocki H (2004) The oxygen stress index and levels of circulating interleukin-10 and interleukin-6 in patients with chronic heart failure. Int J Cardiol 94:283–287. doi:10.1016/j.ijcard.2003.06.001
Thakur S, Gupta N, Kakkar P (2004) Serum copper and zinc concentrations and their relation to superoxide dismutase in severe malnutrition. Eur J Pediatr 163:742–744. doi:10.1007/s00431-004-1517-7
Yang Q, Liu YC, Zou W, Yim AP, He GW (2002) Protective effect of magnesium on the endothelial function mediated by endothelium-derived hyperpolarizing factor in coronary arteries during cardioplegic arrest in a porcine model. J Thorac Cardiovasc Surg 124:361–370. doi:10.1067/mtc.2002.122548
Acknowledgments
The work described in this paper was fully supported by grants from the National Natural Science Foundation of China (no. 81170148), International S & T Cooperation Program of China (no. 2009DFB30560), and the National Basic Research Program of China (no. 2010CB529500), and Tianjin Municipal Science and Technology Commission 09ZCZDSF04200 and 10JCYBJC26400, Tianjin Municipal Research Grant for Applied Basic and Frontier Technology, China, Binhai Key Platform for Creative Research Program (2012-BH110004), Tianjin Binhai New Area Health Bureau ((2011BHKZ001 and 2012BWKZ008) and (2011BHKY002 and 2012BWKY024)), and Hong Kong Research Grants Council Grants (GRF CUHK4789/09M & 4774/12M). The assistance of the surgical team and nurses in the Cardiac Operating Theater, TEDA International Cardiovascular Hospital is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, YQ., Liu, XC., Jing, WB. et al. Alteration of Plasma Trace Elements in Patients Undergoing Open Heart Surgery. Biol Trace Elem Res 151, 344–349 (2013). https://doi.org/10.1007/s12011-012-9577-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9577-4