Abstract
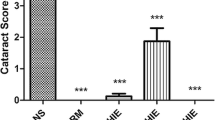
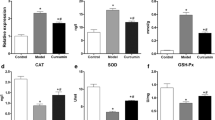
The present investigation is aimed to evaluate the anticataractogenic potential of C-phycocyanin (C-PC), extracted and purified from Spirulina platensis. Enucleated rat lenses were maintained in vitro in Dulbecco’s modified Eagle medium (DMEM). Group I contained DMEM, Group II and Group III contained 100 μM of sodium selenite, Group III was subdivided into three viz IIIa, IIIb, IIIc supplemented with 100, 150, 200 μg of C-PC respectively. In the in vivo study, on tenth day post partum: Group I rat pups received an intraperitoneal injection of saline, Group II, IIIa, IIIb, and IIIc rat pups received a subcutaneous injection of sodium selenite (19 μmol/kg bodyweight) Group IIIa, IIIb, IIIc also received an intraperitoneal injection of 100, 150, 200 mg/kg body weight of C-PC, respectively, from postpartum days 9–14. On termination of the experiment, the lenses from both in vitro and in vivo studies were subjected to morphological examination and subsequently processed to estimate the activities of antioxidant enzymes namely superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, levels of reduced glutathione and lipid peroxidation products. Sodium selenite-exposed, C-PC-treated rat lenses (Group IIIc), showed significant restoration of antioxidant enzyme activity (p < 0.05) when compared to their counterpart Group II. Group IIIc conserved the levels of GSH and lipid peroxidation products at near to normal levels as compared with Group II. Results conclude the possible role of C-PC in modulating the antioxidant enzyme status, thereby retarding sodium selenite-induced cataract incidence both in vitro and in vivo.



Similar content being viewed by others
References
Shearer TR, Ma H, Fukiage C, Azuma M (1997) Selenite nuclear cataract: review of the model. Mol Vis 3:8
Kisic B, Miric D, Zoric L, Ilic A, Dragojevic I (2012) Antioxidant capacity of lenses with age-related cataract. Oxid Med Cell Longev 2012:467130
Peter K, Ratnasamy S (2009) Unifying mechanism for eye toxicity: electron transfer, reactive oxygen species, antioxidant benefits, cell signaling and cell membranes. Society of Cell Membranes and Free Oxygen Radicals. Cell Memb and Free Rad Res. Vol 1, No 2
Kumarasamy A, Abraham EC (2008) Interaction of C-terminal truncated human alphaA-crystallins with target proteins. PLoS One 3:e3175
Halliwell B (2007) Biochemistry of oxidative stress. Biochem Soc Trans 35:1147–1150
Pelizer LH, Danesi EDG, Rangel CO, Sassano CEN, Carvalho JCM, Sato S, Moraes IO (2003) Influence of inoculum age and concentration in Spirulina platensis cultivation. J Food Eng 56:371–375
Wang L, Pan B, Sheng J, Xu J, Hu Q (2007) Antioxidant activity of Spirulina platensis extracts by supercritical carbon dioxide extraction. Food Chem 105:36–41
Pinero Estrada JE, Bermejo Bescos P, Villar del Fresno AM (2001) Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco 56:497–500
Edwards MR, MacColl R, Eisele LE (1996) Some physical properties of an unusual C-phycocyanin isolated from a photosynthetic thermophile. Biochim Biophys Acta 1276:64–70
Bhat VB, Madyastha KM (2000) C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun 275:20–25
Hirata T, Tanaka M, Ooike M, Tsunomura T, Sakaguchi M (2000) Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J Appl Phycol 12:435–439
Cherng SC, Cheng SN, Tarn A, Chou TC (2007) Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci 81:1431–1435
Manconia M, Pendas J, Ledon N, Moreira T, Sinico C, Saso L, Fadda AM (2009) Phycocyanin liposomes for topical anti-inflammatory activity: in-vitro in-vivo studies. J Pharm Pharmacol 61:423–430
Rooban BN, Sasikala V, Sahasranamam V, Abraham A (2010) Vitex negundo modulates selenite-induced opacification and cataractogensis in rat pups. Biol Trace Elem Res 138:282–292
Zigler JS Jr, Qin C, Kamiya T, Krishna MC, Cheng Q, Tumminia S, Russell P (2003) Tempol-H inhibits opacification of lenses in organ culture. Free Radic Biol Med 35:1194–1202
Isai M, Elanchezhian R, Sakthivel M, Chinnakkaruppan A, Rajamohan M, Jesudasan CN, Thomas PA, Geraldine P (2009) Anticataractogenic effect of an extract of the oyster mushroom, pleurotus ostreatus, in an experimental animal model. Curr Eye Res 34:264–273
Boussiba S, Richmond AE (1979) Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch Microbiol 120:155–159
Gupta SK, Kalaiselvan V, Srivastava S, Saxena R, Agrawal SS (2010) Trigonella foenum-graecum (Fenugreek) protects against selenite-induced oxidative stress in experimental cataractogenesis. Biol Trace Elem Res 136:258–268
Anitha TS, Annadurai T, Thomas PA, Geraldine P (2011) Prevention of selenite-induced cataractogenesis by an ethanolic extract of Cineraria maritima: an experimental evaluation of the traditional eye medication. Biol Trace Elem Res 143:425–436
Isai M, Sakthivel M, Ramesh E, Thomas PA, Geraldine P (2009) Prevention of selenite-induced cataractogenesis by rutin in Wistar rats. Mol Vis 15:2570–2577
Cheng C, Xia CH, Huang Q, Ding L, Horwitz J, Gong X (2010) Altered chaperone-like activity of alpha-crystallins promotes cataractogenesis. J Biol Chem 285:41187–41193
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16:359–364
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Staal GE, Helleman PW, de Wael J, Veeger C (1969) Purification and properties of an abnormal glutathione reductase from human erythrocytes. Biochim Biophys Acta 185:63–69
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
David LL, Shearer TR (1984) Calcium-activated proteolysis in the lens nucleus during selenite cataractogenesis. Invest Ophthalmol Vis Sci 25:1275–1283
Rikans LE, Hornbrook KR (1997) Lipid peroxidation, antioxidant protection and aging. Biochim Biophys Acta 1362:116–127
Naziroglu M, Dilsiz N, Cay M (1999) Protective role of intraperitoneally administered vitamins C and E and selenium on the levels of lipid peroxidation in the lens of rats made diabetic with streptozotocin. Biol Trace Elem Res 70:223–232
Varma SD, Ramachandran S, Devamanoharan PS, Morris SM, Ali AH (1995) Prevention of oxidative damage to rat lens by pyruvate in vitro: possible attenuation in vivo. Curr Eye Res 14:643–649
Varma SD, Chand D, Sharma YR, Kuck JF Jr, Richards RD (1984) Oxidative stress on lens and cataract formation: role of light and oxygen. Curr Eye Res 3:35–57
Varma SD, Hegde KR, Kovtun S (2010) Inhibition of selenite-induced cataract by caffeine. Acta Ophthalmol 88:e245–e249
Kyselova Z (2010) Different experimental approaches in modelling cataractogenesis: an overview of selenite-induced nuclear cataract in rats. Interdiscip Toxicol 3:3–14
Hayes JD, McLellan LI (1999) Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31:273–300
Ganea E, Harding JJ (2006) Glutathione-related enzymes and the eye. Curr Eye Res 31:1–11
Cano-Europa E, Ortiz-Butrón R, Gallardo-Casas C, Blas-Valdivia V, Pineda-Reynoso M, Olvera-Ramírez R, Franco-Colin M (2010) Phycobiliproteins from Pseudanabaena tenuis rich in c-phycoerythrin protect against HgCl2 caused oxidative stress and cellular damage in the kidney. J Appl Phycol 22:495–501
Naziroglu M, Karaoglu A, Aksoy AO (2004) Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 195:221–230
Naziroglu M (2009) Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res 34:2181–2191
Naziroglu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32:1990–2001
Manikandan R, Thiagarajan R, Beulaja S, Sudhandiran G, Arumugam M (2010) Curcumin prevents free radical-mediated cataractogenesis through modulations in lens calcium. Free Radic Biol Med 48:483–492
Bermejo-Bescos P, Pinero-Estrada E, Villar del Fresno AM (2008) Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol In Vitro 22:1496–1502
Acknowledgments
The authors gratefully acknowledge the financial assistance from DST-PURSE, ICMR-JRF and DST-INSPIRE in the form of fellowships to the first three authors, respectively. The authors also thank the Department of Science and Technology (DST), New Delhi for the DST-YS project (SR/FT/LS-153/2008/ (2010-2013)) sanctioned to the corresponding author. The authors thank Dr. R. Sarada, Scientist, Department of Plant and Cell Biology, CFTRI, Mysore for providing Spirulina platensis culture and Dr. K. Natarajaseenivasan, Associate Professor and Head, Department of Microbiology, Bharathidasan University for the in vitro culture facility support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumari, R.P., Sivakumar, J., Thankappan, B. et al. C-Phycocyanin Modulates Selenite-Induced Cataractogenesis in Rats. Biol Trace Elem Res 151, 59–67 (2013). https://doi.org/10.1007/s12011-012-9526-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9526-2