Abstract
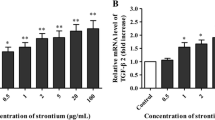
This experiment was conducted to measure the effect of copper supplementation on TGF-β gene expression in chondrocytes of newborn pigs. Chondrocytes were cultured in media containing 15% fetal calf serum supplemented with 0, 15.6, 31.2, and 62.5 μmol/L copper in 90-mm culture plate. Total RNA was isolated from chondrocytes, and TGF-β cDNA was synthesized, amplified, and sequenced. The expression level of TGF-β was examined by reverse transcription polymerase chain reaction. The results showed that the sequence of the cloned TGF-β gene was 99.4% identical to that in GenBank. The expression of TGF-β increased in culture media added with final concentration of 15.6, 31.2, and 62.5 μmol/L copper. In this study, the optimal copper concentration and optimal culture time for the highest level of TGF-β expression were 31.2 μmol/L and 48 h, respectively.




Similar content being viewed by others
References
Braude R (1945) Some observations, on the need for copper in the diet of fattening pigs. J Agric Sci 35:163–167
Li J, Yang L, Zheng X et al (2008) Effect of high dietary copper on weight gain and neuropeptide Y level in the hypothalamus of pigs. J Trace Elem Med Biol 22:33–38
Wanju Li, Yijen Jiang, RockYS Tuan (2006) Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng 12:1775–1885
Yang W, Wang J, Liu L et al (2010) Effect of high dietary copper on somatostatin and growth hormone-releasing hormone levels in the hypothalami of growing pigs. Biol Trace Elem Res. doi:10.1007/s12011-010-8904-x
Prohaska JR, Gybina AA (2004) Intracellular copper transport in mammals. J Nutr 134:1003–1006
Gajewskaa A, Gajkowskab B, Pajakb B et al (2009) Impaired growth hormone-releasing hormone neurons ultrastructure and peptide accumulation in the arcuate nucleus of mosaic mice with altered copper metabolism. Brain Res Bull 80:128–132
Frenkel SR, Saadeh PB, Mehrara BJ et al (2000) Transforming growth factor beta superfamily members: role in cartilage modeling. Plast Reconstr Surg 105:980–990
Aurich M, Anders J, Trommer T et al (2006) Histological and cell biological characterization of dissected cartilage fragments in human osteochondritis dissecans of the femoral condyle. Arch Orthop Trauma Surg 126:606–614
Lind M (1996) Growth factors, possible new clinical tools: a review. Acta Orthop Scand 67:407–417
John MW, Howard JS (2004) Protein-based tissue engineering in bone and cartilage repair. Curr Opin Biotechnol 15:392–398
Bolander ME (1992) Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200:165–170
Tang Y, Wu X, Lei W, Pang L et al (2009) TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15:757–765
Solheim E (1998) Growth factors in bone. Int Orthop 22:410–416
Asanbaeva A, Masuda K et al (2008) Regulation of immature cartilage growth by IGF-I, TGF-β1, BMP-7 and PDGF-AB: role of metabolic balance between fixed charge and collagen network. Biomech Model Mechan 7:263–276
Klisch SM, Chen SS, Sah RL et al (2003) A growth mixture theory for cartilage with applications to growth-related experiments on cartilage explants. J Biomech Eng 125:169–179
Derynck R, Akhurst RJ, Balmain A (2001) TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29:117–129
Joyce ME, Jingushi S, Bolander ME (1990) Transforming growth factor-β in the regulation of fracture repair. Orthop Clin NAM 21:199–209
Tchetina E, Mwale F, Poole AR (2003) Distinct phases of coordinated early and late gene expression in growth plate chondrocytes in relationship to cell proliferation, matrix assembly, remodeling and cell differentiation. J Bone Miner Res 18:844–851
Guowen Liu, Haihua Feng, Zhe Wang (2005) Effect of copper on proliferation and cellular skeleton in the young pig chondrocytes in vitro. Animal Husbandry and Veterinary Medicine 37:13–16
Gerard CB, William PS, Harvey FL (2000) Role of transforming growth factor β in human disease. N Engl J Med 342:1350–1358
Ramoshebi LN, Matsaba TN, Teare J (2002) Tissue engineering: TGF-β super-family members and delivery systems in bone regeneration. Expert Rev Mol Med 4:1–11
Leem K, Park SY, Lee DH et a1 (2003) Effects ol Jaoga-Yukmiwon(R), a Korean herbal medicine, on chondrocyte proliferation and longitudinal bone growth in adolescent male rats. Phytother Res 17:1113–1116
Poustie MW, Carran J, McEleney K et al (2004) N-Butyryl glucosamine increases matrix gene expression by chondrocytes. J Pharmacol Exp Ther 311:610–616
Derynk R, Jarrell JA, Chen EY et a1 (1985) Human transforming growth factor-beta cDNA sequence and expression in normal and transformed cells. Nature 316:701
Quinn JM, Itoh K, Udagawa N et al (2001) Transforming growth factor β affects osteoclast differentiation via direct and indirect actions. J Bone Miner Res 16:1787–1794
Jong Eun Lee, Seoung Eun Kim, Ick Chan Kwon et al (2004) Effects of a chitosan scaffold containing TGF-β1 encapsulated chitosan microspheres on in vitro chondrocyte culture. International Center for Artificial Organs and Transplantation 28:829–839
Ionescu AM,Achwarz EM,Zuscik MJ et a1 (2003) ATF-2 cooperates with Smad3 to mediate TGF-beta effects on chondrocyte maturation. Exp Cell Res 288:198–207
Noda M (1988) Transcriptional regulation of osteopontin production in rat osteosarcoma cell by type beta transforming growth factor. J Biol Inorg Chem 265:391
Flanders KC, Bhandiwad AR, Winokur TS (1997) Transforming growth factor-betas block cytokine induction of catalase and xanthine oxidase mRNA levels in cultured rat cardiac ce11s. J Mol Cell Cardiol 29:273–280
Roman-Blas JA, Stokes DG, Jimenez SA (2007) Modulation of TGF-β signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthritis Cart 15:1367–1377
McCormack MC, Nowak KC, Koch RJ (2001) The effect of copper tripeptide and tretinoin on growth factor production in a serum-free fibroblast model. Arch Facial Plast Surg 3:28–32
Grimaud E, Heymann D, Redini F (2002) Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev 13:241–257
Maquart FX, Pickart I (1988) Stimulation of collagen synthesis in fibrolast cultures by the tripeptide–copper complex glycyl-L-histidyl-I-lysine-Cu. Febs Lett 238: 343–346
Buckwalter JA, Mankin HJ (1997) Articular cartilage. Part I. Tissue design and chondrocyte matrix interactions: Part II. Degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg AM 79A:600–632
Aristidis Moustakas, Katerina Pardali, Annamaria Gaal et al (2002) Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol Lett 82:85–91
Rodriguez JP, Susana Rios, Mauricio Gonzalez (2002) Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J Cell Biochem 85:92–100
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant nos. 31072178, 30600441, 30972212, and 30871897), by the Doctoral Program Foundation of Institutions of Higher Education of China (Grant no. 20070183156), and by the Science Frontier and Cross-disciplinary Innovation Project of Jilin University (Grant no. 200903022).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiaoyan Zhu, Jianguo Wang, Haihua Feng, and Guanghong Xie contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhu, X., Wang, J., Xie, G. et al. Effect of Copper on the Expression of TGF-β in Incubated Chondrocytes of Newborn Pigs. Biol Trace Elem Res 143, 1461–1469 (2011). https://doi.org/10.1007/s12011-011-8966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-8966-4