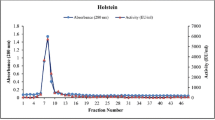
Abstract
Paraoxonase-1 (PON1) is an organophosphate hydrolyser enzyme which has also antioxidant properties in metabolism. Due to its crucial functions, inhibition of the enzyme is undesirable and very dangerous. PON1 enzyme activity should not be altered in any case. Inhibitory investigations of this enzyme are therefore important and useful. Metal toxicology of enzymes has become popular in the recent years. Here, we report the in vitro inhibitory effects of some metal ions, including Pb+2, Cr+2, Fe+2, and Zn+2, on the activity of human serum PON1 (hPON1; EC 3.1.8.1.). For this purpose, we purified the enzyme from human serum and analyzed the alterations in the enzyme activity in the presence of metal ions. The results show that metal ions exhibit inhibitory effects on hPON1 at low concentrations with IC 50 values ranging from 0.838 to 7.410 mM. Metal ions showed different inhibition mechanisms: lead and iron were competitive, chrome was noncompetitive, and zinc was uncompetitive. Lead was determined to be the most effective inhibitor.




Similar content being viewed by others
Abbreviations
- PON1:
-
Paraoxonase-1
- OPs:
-
Organophosphates
References
Draganov DI, La Du BN (2004) Pharmacogenetics of paraoxonases: a brief review. Naunyn-Schmiedeberg’s Arch Pharmacol 369:78–88
Shih DM, Gu L, Xia YR et al (1998) Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 394:284–287
Rozenberg O, Rosenblat M, Coleman R et al (2003) Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic Biol Med 34:774–784
Tward A, Xia YR, Wang XP et al (2002) Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 106:484–490
Oda MN, Bielicki JK, Ho TT et al (2002) Paraoxonase 1 overexpression in mice and its effect on high-density lipoproteins. Biochem Biophys Res Commun 290:921–927
Jarvik GP, Rozek LS, Brophy VH et al (2000) Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1(192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol 20:2441–2447
Mackness B, Davies GK, Turkie W et al (2001) Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol 21(9):1451–1457
Mackness B, Durrington P, McElduff P et al (2003) Low paraoxonase activity predicts coronary events in the Caerphilly prospective study. Circulation 107(22):2775–2779
Ng CJ, Wadleigh DJ, Gangopadhyay A et al (2001) Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem 276:44444–44449
Reddy ST, Wadleigh DJ, Grijalva V et al (2001) Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol 21:542–547
Nga CJ, Shiha DM, Hamaa SY et al (2005) The paraoxonase gene family and atherosclerosis. Free Radic Biol Med 38:153–163
Aviram M, Rosenblat M, Bisgaier CL et al (1998) Paraoxonase inhibits highdensity lipoprotein oxidation and preserves its functions. a possible peroxidative role for paraoxonase. J Clin Invest 101:1581–1590
Deakin S, James R (2004) Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci 107:435–447
Hu H, McCally M (eds) (2002) Life support: the environment and human health (book review). J Sociol Soc Welf 4:1–9
Hura C, Hura BA (2006) Assessment of the heavy metals in the food from Romania, 2005. Misc Toxicol Lett 164:270
Renault F, Chabrière E, Andrieu JP et al (2006) Tandem purification of two HDL-associated partner proteins in human plasma, paraoxonase (PON1) and phosphate binding protein (HPBP) using hydroxyapatite chromatography. J Chromatogr B 836:15–21
Sinan S, Kockar F, Arslan O (2006) Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglycoside derived antibiotics. Biochimie 88:565–574
Ekinci D, Senturk M, Beydemir S, Kufrevioglu OI (2008) An alternative method for purifying human serum paraoxonase-1, IV. National Affinity Techniques Congress, 4–7 May, p 61
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–251
Laemmli DK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–683
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 57:685
Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R (2005) Low level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113:894–899
Eisler R (1993) Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. Contaminant Hazard Reviews. Report 26
Ekinci D, Beydemir S, Kufrevioglu OI (2007) In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 22:745–750
Coban TA, Senturk M, Ciftci M et al (2007) Effects of some metal ions on human erythrocyte glutathione reductase: an in vitro study. Protein Pept Lett 14:1027–1030
Tekman B, Ozdemir H, Senturk M, Ciftci M (2008) Purification and characterization of glutathione reductase from rainbow trout (Oncorhynchus mykiss) liver and inhibition effects of metal ions on enzyme activity. Comp Biochem Physiol C 148:117–121
Soyut H, Beydemir S, Hisar O (2008) Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol Trace Elem Res 123:179–190
Pla A, Rodrigo L, Hernandez AF et al (2007) Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem Biol Interact 167:63–70
Gencer N, Arslan O (2009) Purification human PON1(Q192) and PON1(R192) Isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chromatogr B 877:134–140
Hernández AF et al (2009) Interaction between human serum esterases and environmental metal compounds. Neurotoxicology 30(4):628–635
Chait A, Han C, Oram J et al (2005) The immune system and atherogenesis. lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res 46:389–403
Kasprzak M, Iskra M, Majewski W et al (2009) Arylesterase and paraoxonase activity of paraoxonase (PON1) affected by ischemia in the plasma of patients with arterial occlusion of the lower limbs. Clin Biochem 42:50–56
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekinci, D., Beydemir, Ş. Purification of PON1 from Human Serum and Assessment of Enzyme Kinetics Against Metal Toxicity. Biol Trace Elem Res 135, 112–120 (2010). https://doi.org/10.1007/s12011-009-8500-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-009-8500-0