Abstract
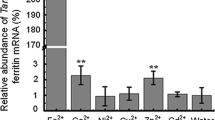
The ability of iron to accept and donate electrons makes it important for plant growth, but it can also damage plants when they are under environmental stress. Ferritin, a protein encoded by the gene Fer, catalyzes the oxidation of Fe2+ and subsequent storage of Fe3+ within the mineral core. Ferritin may reduce the adverse effects of iron on Chorispora bungeana Fisch. & C.A. May during the course of cold stress. C. bungeana is a rare alpine subnival plant species that is highly resistant to a freezing environment. We have isolated and characterized the ferritin cDNA (CbFer) from C. bungeana. It is 975 bp in length with an open reading frame of 260 amino acids, corresponding to a protein of predicted molecular mass of 29.17 kDa and an isoelectric point of 5.44. Amino acid analysis of the polypeptides indicated that CbFer codes for a ferritin subunit plus a chloroplast-targeting transit peptide. Reverse transcription polymerase chain reaction analysis confirmed that CbFer was a tissue-specific gene since the expression could only be detected in leaves. The gene expression patterns were investigated in relation to cold stress (4°C and −4°C) and to various exogenous signals, including excessive iron, hydrogen peroxide (H2O2), and nitrogen monoxidum (NO). The amount of CbFer mRNA increased in response to low temperatures and gene expression at −4°C was both more distinct and quicker than that at 4°C. Two exogenous signals, excessive iron and H2O2, upregulated the expression of the CbFer gene, but NO had no effect. The CbFer gene may play an important role in response to cold stress, while the expression of the gene during stress may be influenced by major and minor factors such as iron and H2O2, respectively.











Similar content being viewed by others
References
Guy CL, Niemi KJ, Brambl R (1985) Altered gene expression during cold acclimation of spinach. Proc Natl Acad Sci U S A 82:3673–3677
Sala JM, Lafuente MT (2004) Antioxidant enzymes activities and rindstaining in ‘Naveline’ oranges as affected by storage relative humidity and ethylene conditioning. Postharvest Biol Technol 31:277–285
Sairam RK, Srivastava GC, Saxena DC (2000) Increased antioxidant activity under elevated temperature: a mechanism of heat stress tolerance in wheat genotypes. Biol Plant 43:245–251
Jin J, Shan N, Ma N, Bai J, Gao J (2006) Regulation of ascorbate peroxidase at the transcript level is involved in tolerance to postharvest water deficit stress in the cut rose (Rosa hybrida L.) cv. Samantha. Postharvest Biol Technol 40:236–243
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta YK (2002) Yoshimura regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Gilmour SJ, Hajela RK, Thomashow MF (1988) Cold acclimation in Arabidopsis thaliana. Plant Physiol 87:745–750
Hariyadi P, Parkin KL (1991) Chilling-induced oxidative stress in cucumber fruits. Postharvest Biol Technol 1:33–45
Hidey E, Bjorn LO (1996) Ultraweak light emission, free radicals chilling and light sensitivity. Physiol Plant 98:223–228
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage, function and cellular regulation. Biochim Biophys Acta 1275:61–203
Bienfait HF, Van der Mark F (1983) PhytoFerritin and its role in iron metabolism. In: Robb DA, Pierpoint WS (eds) Metals and micronutrients: uptake and utilization by plants. Academic, New York, pp 111–123
Lescure AM, Proudhon D, Pesey H, Ragland M, Theil EC, Briat JF (1991) Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci U S A 88:8222–8226
Petit JM, Briat JF, Lobréaux S (2001) Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem J 359:575–582
Fobis-Loisy I, Loridon K, Lobréaux S, Lebrun M, Briat JF (1995) Structure and differential expression in response to iron and abscisic acid of two maize Ferritin genes. Eur J Biochem 231:609–619
Wardrop AJ, Wicks RE, Entsch B (1999) Occurrence and expression of members of the ferritin gene family in cowpeas. Biochem J 337:523–530
An LZ, Liu YH, Feng HY, Feng GN, Chen GD (2000a) Studies on characteristics of element contents of altifrigetic subnival vegetation at the source area of Urumqi River. Acta Bot Boreal-Occident Sinica 20:80–86
An LZ, Liu YH, Feng GN, Feng HY, Chen GD (2000b) Studies on characteristics of communities in the source region of Urumqi River. Acta Bot Boreal Occident Sinicb 20:80–86
Ayitu R, Tan DY, Li JZ, Fang Y (1998) The relationship between the structure of vegetative organs in C. bungeana and its environment. J Xinjiang Agric Univ 21:273–277
Fu XY, Chang JF, An LZ, Zhang MX, Xu Sj, Chen T, Liu YH, Xin H, Wang JH (2006) Association of the cold-hardiness of C. bungeana with the distribution and accumulation of calcium in the cells and tissues. Environ Exp Bot 55:282–293
Zhang LJ, Yang TW, Gao YS, Liu YB, Zhang TG, Xu SJ, Zeng FL, An LZ (2006) Effect of lanthanum ions (La3+) on ferritin-regulated antioxidant process under PEG stress. Bio Trace Ele Res 113:193–208
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Evans PJ, Halliwell B (1994) Measurement of iron and copper in biological systems: bleomycin and copper-phenanthroline assays. Methods Enzymol 233:82–92
Briat JF, Lobréaux S (1997) Iron transport and storage in plants. Trends Plant Sci 2:187–193
Emanuelsson O, Nielsen H, Von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
Briat JF, Lobréaux S, Grignon N, Vansuyt G (1999) Regulation of plant ferritin synthesis: how and why? Cell Mol Life Sci 56:155–166
Van Wuytswinkel O, Briat JF (1995) Conformational changes and in vitro core formation modifications induced by site-directed mutagenesis of the specific N-terminus of pea seed ferritin. Biochem J 305:959–965
Jaakola L, Pirtila AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotech 19:203–210
Walker K, Croteau R (2000) Taxol biosynthesis–molecular cloning of a benzoyl-CoA-taxane 2a-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci U S A 97:13591–13596
Magdalena G, Lorenzo L (2005) Nitric oxide and iron in plants: an emerging and converging story. Trends Plant Sci 10:4–8
Murgia I, Delledonne M, Soave C (2002) Nitric oxide mediates iron-induced ferritin accumulation in Arabidopsis. Plant J 30:521–528
Guo FX, Zhang MX, Chen Y, Zhang WH, Xu SJ, Wang JH, An LZ (2006) Relation of several antioxidant enzymes to rapid freezing resistance in suspension cultured cells from alpine Chorispora bungeana. Cryobiology 52(2):341–350
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Acknowledgments
The authors thank anonymous reviewers and Dr. Peter Long for their constructive suggestions on the manuscript. This study was supported by the National Basic Research Program of China (973; 2007CB108900) and the National Science Foundation of China (No. 30800801).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Si, J., Zeng, F. et al. Molecular Cloning and Characterization of a Ferritin Gene Upregulated by Cold Stress in Chorispora bungeana . Biol Trace Elem Res 128, 269–283 (2009). https://doi.org/10.1007/s12011-008-8275-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8275-8