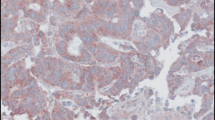
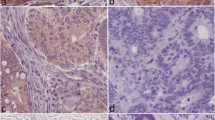
Abstract
IGFLR1 is a novel biomarker, and some evidences suggested that is involved in the immune microenvironment of CRC. Here, we explored the expression of IGFLR1 and its association with the prognosis as well as immune cell infiltration in CRC, with the aim to provide a basis for further studies on IGFLR1. Immunohistochemical staining for IGFLR1, TIM-3, FOXP3, CD4, CD8, and PD-1 was performed in eligible tissues to analyze the expression of IGFLR1 and its association with prognosis and immune cell infiltration. Then, we screened colon cancer samples from TCGA and grouped patients according to IGFLR1-related genes. We also evaluated the co-expression and immune-related pathways of IGFLR1 to identify the potential mechanism of it in CRC. When P < 0.05, the results were considered statistically significant. IGFLR1 and IGFLR1-related genes were associated with the prognosis and immune cell infiltration (P < 0.05). In stage II and III CRC tissue and normal tissue, we found (1) IGFLR1 was expressed in both the cell membrane and cytoplasm and which was differentially expressed between cancer tissue and normal tissue. IGFLR1 expression was associated with the expression of FOXP3, CD8, and gender but was not associated with microsatellite instability. (2) IGFLR1 was an independent prognostic factor and patients with high IGFLR1 had a better prognosis. (3) A model including IGFLR1, FOXP3, PD-1, and CD4 showed good prognostic stratification ability. (4) There was a significant interaction between IGFLR1 and GATA3, and IGFLR1 had a significant co-expression with related factors in the INFR pathway. IGFLR1 has emerged as a new molecule related to disease prognosis and immune cell infiltration in CRC patients and showed a good ability to predict the prognosis of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high morbidity and mortality of colorectal cancer (CRC) patients has brought the attention of the research community [1, 2]. Stage II and III CRC are prognostically heterogeneous due to differences in the molecular phenotypes [3]. Recent studies have shown that the immune microenvironment plays an important role in the prognosis and treatment response of CRC patients [4, 5]. Therefore, exploring the molecular phenotype and immune microenvironment characteristics of tumors is important for the success of personalized therapy. Immunotherapy is regarded as an important therapeutic strategy for CRC patients; however, anti-tumor immunity is blocked by several inhibitory factors. Therefore, blocking the transmission of inhibitory signals is an important strategy in immunotherapy [6]. At present, the blockade of the check-point proteins, PD-1, PD-L1, and CTLA4 has become a common immunotherapy strategy that has achieved good results in malignant tumors [7], but they are prone to therapeutic resistance [8]. Therefore, there is an urgent need to identify novel biomarkers and immune targets. In recent years, insulin growth factor-like receptor 1 (IGFLR1) has been found to play an important role in the CRC and is known to be associated with the transmission of costimulatory signals [9, 10]. In this study, we focused on the expression pattern, prognostic value, molecular mechanism of IGFLR1, and its association with the immune microenvironment. We hope to provide a basis for further studies of IGFLR1.
Methods
Data Collection
All the CRC patients included in this study met the following conditions: (1) they received radical surgical treatment for CRC at the Harbin Medical University Cancer Hospital between January 2013 and December 2013, and were pathologically diagnosed as stage II and III CRC and (2) had a completed record of their follow-up. The exclusion criteria were as follows: (1) received neoadjuvant chemotherapy; (2) recurrence or metastasis within 3 months after surgery; and (3) presence of other cancers at initial diagnosis. Finally, 270 patients were included.
The following information regarding the patients was obtained from the electronic medical record system (EMRS) and the telephone follow-up databases of the Harbin Medical University Cancer Hospital: (1) gender, age, height, weight, smoking history, drinking history, and other individual characteristics; (2) disease information, such as concomitant diseases, tumor sites, surgery time and approaches, postoperative pathological reports, and available CEA values; (3) follow-up information. The pathological stage was defined according to the criteria in the 8th American Joint Committee on Cancer (AJCC) manual for CRC.
Immunohistochemical Staining (IHC)
IHC staining was performed on 270 cancer tissues and 50 normal tissues. The IHC workflow was carried out as previously described [11]. The main experimental equipment and reagents are shown in Table S1.
IHC Grading Criteria
Two experienced pathologists were blinded to score the expression levels of IGFLR1, TIM-3, FOXP3, PD-1, CD4, and CD8 in tissue:
-
(1)
The proportion of positively stained cells: 0, <5%; 1, 5 to 25%; 2, 26 to 50%; 3, 51 to 75%; 4, 76 to 100%.
-
(2)
The staining intensity: 0, no signal; 1, weak; 2, moderate; 3, strong.
-
(3)
The above two scores were then multiplied together to obtain the expression levels. For IGFLR1, the best cutoff value was determined by the “survminer” package in R, where a score of 0 was defined as negative, and ≥1 was defined as positive; for TIM-3, FOXP3, PD-1, CD4, and CD8, the cutoff values used semi-quantitative analysis [12, 13], where score of 0 was defined as negative, score ranging from 1 to 4 was defined as low expression (+), score ranging from 5 to 8 was defined as moderate expression (++), and between 9 and 12 was defined as high expression (+++) [14, 15].
Statistical Analysis
R software (R version 4.0.3) was used for the statistical analysis, and the study endpoints were disease-free survival (DFS), cancer-specific survival (CSS), and overall survival (OS). Kendall’s correlation test was used to identify the correlation between IGFLR1 and other factors. Survival conditions were described by Kaplan-Meier (K-M) survival analysis. Univariate and multivariate Cox regression analyses were used to describe the prognostic value. P < 0.05 was considered statistically significant. In order to build a molecular phenotype-based prognostic model, univariate COX analysis was used to identify the molecules that might be part of the model. To incorporate more possible variables, we considered variables with P < 0.2 to be meaningful and then adopted least absolute shrinkage and selection operator (LASSO) Cox regression to build the final prognostic stratification model (P < 0.05). Finally, the receiver operating characteristic (ROC) curve and area under curve (AUC) were used to test predictive performance of the model. AUC > 0.9 indicated a strong predictive ability; AUC between 0.7 and 0.9 indicated a good predictive ability; and AUC values between 0.5 and 0.7 suggested a moderate predictive ability [16].
Bioinformatics Analysis
Data Collection and Processing
Transcriptome data from 446 colon cancer samples from the TCGA (https://portal.gdc.cancer.gov/) dataset was analyzed in this study, and further information on clinical staging and survival information was also included. The gene IDs were converted into corresponding gene symbols according to the annotation package corresponding to the dataset. In the analysis, data for which expressed RNA could not be detected in >10% of samples were excluded.
Preliminary Screening of IGFLR1-Related Genes
The R package “maxstat” was used to calculate the best cutoff value, and then, all samples were divided into high- and low-expression groups [17]. The R package “Limma” was used to analyze differentially expressed genes (DEGs) in the two groups (|log2foldchange| > 0.5, P-adj < 0.05) [18]. The up- and downregulated DEGs in the high expression group were visualized using volcano plots. The R package “ESTIMATE”, an algorithm based on the ssGSEA scores of the expression matrix of each tumor sample by using two related gene sets (stromal and immune), was used to evaluate immune infiltration in the TCGA samples [19].
Construction of IGFLR1-Related Gene Co-expression Modules
We used weighted gene co-expression network analysis (WGCNA) to establish consensus gene modules of IGFLR1-related genes and analyzed the correlation between the modules and the “ESTIMATE” calculation results [20]. At first, we utilized the power function to construct the adjacency matrix (AM). Then by selecting an appropriate power index to increase the similarity of matrix, a scale-free co-expression network was constructed. Subsequently, the AM was transformed into a topological overlap matrix (TOM). We performed average linkage hierarchical cluster analysis based on dissimilarity measurements of TOM. Among them, the correlation coefficient (CC) was defined as the magnitude of the correlation between the eigengenes of each module and the score. Gene significance (GS) was defined as P-value in linear regression analysis of expression and score for each gene (GS = lgP). Finally, we obtained gene clustering tree, co-expression modules, and corresponding correlations.
Identifying IGFLR1-Related Immune Genes and Building IGFLR1-Related Models
To further identify immune genes associated with IGFLR1, we selected the module with the highest correlation coefficient with the immune score and calculated GS and module membership (MM) in this module. MM is a measure of the connectivity of genes and modules. We defined the screening thresholds as “cor. gene MM > 0.5 and cor. gene GS > 0.5.” We performed univariate Cox proportional hazards regression analysis on these genes screened from the module. Genes with P < 0.05 in the univariate Cox analysis were then retained for subsequent Lasso-Cox proportional hazards regression with 1000 simulations by the R package “glmnet” [21]. Receiver operating characteristics (ROC) at years 1, 3, and 5 were plotted using the R package “survival ROC” and the area under the curve (AUC) was determined [22].
Evaluation of the Predictive Performance of the Model and Analysis of Differences in Immune Infiltration Between High- and Low-Risk Groups
First, we used principal component analysis (PCA) to perform dimensionality reduction cluster analysis on the genes in the model to verify its discriminating effect on high- and low-risk groups. Log-rank test was used to analyze the prognosis of patients in high- and low-risk groups to test the predictive value of the model for determining prognosis. Then, the risk score was combined with other clinical factors for univariate and multivariate Cox regression analysis to further verify the independent effect of the risk score.
To analyze differences in immune infiltration between high- and low-risk groups, we used another algorithm to estimate the relative infiltrating abundance of 22 immune cells in different samples by R package “CIBERSORT” [23]. Differential analysis was performed on the content of various immune cells in the high- and low-risk groups by the Wilcoxon rank sum test. The results of the differences in the infiltration of these immune cells were displayed using radar charts.
Immune Infiltration Score and Signaling Pathway Analysis
To explore the relationship between IGFLR1 and immune cell infiltration, we used the following methods: Tumor Immune Estimation Resource (TIMER) databases (https://cistrome.shinyapps.io/timer/), Estimating the Proportion of Immune and Cancer cells (EPIC) (https://gfellerlab.shinyapps.io/EPIC_1-1/), and Cell-type Identification By Estimating Relative Subsets Of RNA Transcripts (CIBERSORT) (http://cibersortx.stanford.edu). TIMER showed levels of six tumor-infiltrating immune subsets including B cells, CD4+T cells, CD8+T cells, macrophages, neutrophils, and dendritic cells, which was precalculated for 10,897 tumors from 32 cancer types. It provided six major analytic modules that allowed users to interactively explore the associations between immune infiltrates and a wide spectrum of factors, including gene expression, clinical outcomes, somatic mutations, and somatic copy number alterations. Immune infiltration inferred by TIMER was more strongly correlated with patient clinical characteristics, viral infection status, and cancer gene variants than other computational methods [24]. EPIC could accurately detect the major cell types in a tumor directly based on the gene expression levels in the tumor. The number of cells in tumor could be predicted by comparing the expression levels of genes in a tumor sample with a library of tumor-specific cell type-specific expression profiles. EPIC integrated novel gene expression profiles of major nonmalignant cell types in tumors while also accounting for uncharacterized and potentially highly variable cell types [25]. CIBERSORT estimated the abundance of immune cells by deconvoluting the expression matrix of immune cell subtypes. CIBERSORT provided 22 common immune infiltrating cell expression data (LM22), including immune cells of different cell types and functional states, enabling large-scale analysis of RNA mixtures of cellular biomarkers and therapeutic targets [23, 26].
Differential analysis was performed on the contents of various immune cells in high- and low-risk groups by the Wilcoxon rank sum test. The results of the differences in the infiltration of these immune cells were displayed using radar charts. Immune-related genes were downloaded from the TISIDB database (http://cis.hku.hk/TISIDB), and then the correlations between IGFLR1 with immune-related genes were calculated. Subsequently, we performed co-expression analysis of IGFLR1 and genes from immune-related pathways which were collected from the ImmPort database (https://www.immport.org/) to identify the mechanism associated with IGFLR1. P < 0.05 was considered significantly associated.
Results
Patient Characteristics
We included 270 stage II and III CRC patients, and the average age of the patients was 60 years (20–83 years). There were 141 patients (52.22%) with stage II, and 129 patients (47.78%) with stage III. The last follow-up date was December 11, 2019, and the median follow-up time was 2198 days (Table S2).
Expression of IGFLR1 in Stage II and III CRC Patients
Previous studies have confirmed that IGFLR1 is highly expressed in CXCL13+BHLHE40+TH1-like cells, CD8+Tex cells, and Treg cells [9, 10]. Therefore, based on the results and conclusions from previously published studies, we performed IHC staining for IGFLR1, TIM-3, FOXP3, PD-1, CD4, and CD8. After excluding slides with quality problems, 270 slides were stained for IGFLR1 and 206 were stained for all six candidate markers. In addition, we were also staining for IGFLR1 in normal colorectal tissues (n = 50), the purpose was to explore the expression of IGFLR1. In stage II and III CRC, IGFLR1 was expressed in the cell membrane and cytoplasm, and positive staining rate was 42.22% (Fig. 1). In normal colorectal tissues, IGFLR1 was expressed in the cell membrane and cytoplasm, which positive staining rate was 58% (Fig. S2D top). The differential analysis result showed that IGFLR1 was differentially expressed in cancer tissue and normal tissue (Fig. S2D bottom). The expression levels of the above proteins are shown in Table S3 (Table S3). Following this, 28 MSI-H CRC patients and 28 MSS CRC patients were compared, and we found no significant difference in the expression level of IGFLR1 across the different microsatellite subtypes (P = 0.649) (Table S4).
We further explored the correlation between IGFLR1 and other factors by combining the clinical information and pathological information. Kendall’s correlation test confirmed that IGFLR1 was associated with FOXP3 (r = 0.145, 95% CI 0.009–0.277, P = 0.037), CD8 (r = 0.167, 95% CI 0.031–0.297, P = 0.016), and gender (r = 0.003, 95 % CI −0.334 to −0.072, P = 0.003) (Table S5).
IGFLR1 Expression Was Associated With the Prognosis for Stage II and III CRC Patients
Using K-M survival analysis, we evaluated the prognostic significance of IGFLR1 for stage II and III CRC patients. The analysis found that IGFLR1 expression had a major impact on patient prognosis. IGFLR1-positive patients had better OS (P = 0.011), DFS (P = 0.013), and CSS (P = 0.027) than IGLFR1-negative patients (Fig. 2A, Fig. S2A).
Association between IGFLR1 and prognosis and development of a prognostic model based on IGFLR1. A Overall survival (OS) and disease-free survival (DFS) of CRC patients based on the expression levels of IGFLR1. B ROC curves evaluating the predictive ability of the independent prognostic factor. C LASSO regression model for the construction of a prognostic stratification model. D Molecular expression data of patients from each group. E Overall survival (OS) and disease-free survival (DFS) of different groups after cluster analysis. F ROC curves evaluating the predictive ability of the prognostic stratification model
To further explore the prognostic value of IGFLR1 in stage II and III CRC patients, we performed univariate and multivariate COX regression analysis on patients with complete clinical and molecular expression information. Univariate COX regression analysis found that IGFLR1, N stage, AJCC stage, neural invasion, CEA level, intravascular tumor thrombus, tumor location, and CD4 expression were prognostic factors for stage II and III CRC patients (all values of P were < 0.05). Stepwise multivariate Cox regression analysis revealed that IGFLR1 (HR = 0.580, 95% CI 0.355–0.947, P = 0.0294), N stage, neural invasion, CEA level, and tumor location were all independent prognostic factors for stage II and III CRC patients (all values of P were < 0.05). (Table S6, Table S7).
The ROC curve indicated that the AUC of the above independent prognostic factors in predicting the 3-year and 5-year survival was 0.75 and 0.78, the sensitivity was 0.75 and 0.60, and the specificity was 0.67 and 0.86, respectively, which showed that the above factors had a good predictive ability for the prognosis of stage II and III CRC patients (Fig. 2B). But ROC also showed that the prognostic performance of IGFLR1 was worse than classical markers such as CEA and CA199 (Fig. S2B).
Prognostic Stratification Model Based on Immune Cell Infiltration Characteristics
To stratify patients more accurately, we constructed a prognostic stratification model which included IGFLR1. Firstly, IGFLR1, FOXP3, TIM-3, PD-1, CD4, and CD8 were screened by univariate COX analysis, and the variables with P < 0.2 were further analyzed. Finally, five variables were selected: IGFLR1, FOXP3, PD-1, CD4, and CD8 (Table S8). And through LASSO regression analysis, the stratification model characterized as IGFLR1, FOXP3, PD-1, and CD4 was obtained (Fig. 2C). Based on this model, we divided patients into three groups: patients in group A had a high expression of CD4 and FOXP3, patients in group B had a low expression of IGFLR1, FOXP3, PD-1, and CD4, and patients in group C had a high expression of PD-1 and CD4. The molecular expression data of each group are shown in Table S9 and Fig. 2D. Then the K-M survival analysis showed that the OS (P = 0.009), DFS (P = 0.0077), and CSS (P = 0.089) of patients in group B were worse than those in groups A and C. However, the differences in CSS were not statistically significant (Fig. 2E, Fig. S2C).
To further evaluate the predictive ability of the model, we used ROC curves and AUC. It was found that when predicting the prognosis of patients at 3 and 5 years, the model had an AUC of 0.76 and 0.71, a sensitivity of 0.81 and 0.68, and a specificity of 0.60 and 0.64, respectively. The results suggested that the above model had a good predictive ability for the prognosis of patients with stage II and III CRC and could predict short-term prognosis with a higher sensitivity and specificity (Fig. 2F).
Screening of IGFLR1-Related Genes
Then, we used bioinformatics database to further explore and verify the expression and function of IGFLR1. We first screened IGFLR1-related genes in the TCGA database. And the best cutoff value was estimated by R package “maxstat” [17]. Then, we analyzed the differentially expressed genes (DEGs) of the two groups (| log2 fold change | & gt; 0.5, P - adj & lt; 0.05) (Fig. 3A). We further evaluated the correlation between the above genes and immune-related genes using WGCNA. Immune-related genes with the same expression pattern were clustered (Fig. 3B–E) and divided into 5 modules. The correlation score of each module is shown in Fig. 3D. The stromalscore, immunescore, and ESTIMATE score of MEblue were 0.81 (3e−120), 0.87 (1e−140), and 0.9 (3e−158), respectively, which suggested the related genes of this module had the highest correlation with immune infiltration. Figure 3F also suggested that there was a significant correlation between immune infiltration and MEblue.
Screening of IGFLR1-related genes and construction of immune infiltration-related models. A Analysis of differential gene expression of different groups. B–E Cluster analysis of gene expression pattern. F Correlation between different cluster groups and immune infiltration. G, H LASSO analysis was used to screen prognostic genes
Construction of a Prognosis Risk Model Based on Genes in MEblue
The genes in MEblue were used to construct prognostic model. Univariate Cox regression analysis and LASSO regression analysis were used for screening prognostic genes and the included genes were RAB42, C1QA, CD14, APOE, C1QC, SIGLEC1, C1QB, GPR141, PPM1M, CD300A, and TMIGD3. The variables with P < 0.05 were further analyzed. RAB42, C1QA, CD14, APOE, C1QC, SIGLEC1, C1QB, GPR141, PPM1M, and CD300A were obtained and finally be included in the model (Table S10).
The Model Showed Good Ability to Stratify Patients into Different Risk Groups
According to the model, the patients were divided into high and low-risk groups (Fig. 4A, B). Firstly, we performed immune infiltration analysis in two groups, and found that CD4 and memory T cells were highly expressed in the low-risk group, while CD8 and Treg cells were highly abundant in the high-risk group (Fig. 5G–J). Next, we performed prognostic analysis. The results showed that patients in the high-risk group had a worse prognosis than those in the low-risk group (P < 0.001) (Fig. 4C). To further verify the relationship between the model and prognosis, we included the risk score of the model as a related factor in univariate and multivariate Cox regression analysis. We found that the risk score of the model could be used as an independent prognostic factor for colon cancer ((HR = 2.531; 95% CI, 1.770–3.619, P < 0.001) (Fig. 4D, E). The ROC curves and AUC were used to describe the predictive efficacy of the model. The results showed that the 1, 3, and 5-year AUC values were 0.706, 0.7.8, and 0.725, respectively. The results suggested that the model had a good ability to predict the prognosis of patients (Fig. 4F).
Construction of prognostic model based on IGFLR1-related genes and analysis of immune infiltration in different risk groups. A, B Stratification of patients into high and low-risk groups. C Kaplan-Meier curves for the overall survival (OS) between low- and high-risk groups. D, E Univariate and multivariate analysis for predicting factors. F Receiver operating characteristic (ROC) curves and area under curve (AUC) for the prognostic model. G–J Analysis of immune infiltration in high- and low-risk groups
IGFLR1 Was Correlated With Immune Cell Infiltration
We used three bioinformatics methods to score immune infiltration, namely, TIMER, EPIC, and CIBERSHOT. We found that the expression of IGFLR1 was positively associated with the immune cell infiltration in breast cancer (BRCA), lung adenocarcinoma (LUAD), and testicular cancer (TGCT), while it was negatively associated with gastric adenocarcinoma (STAD) (Pearson correlation analysis, P < 0.05). The results from the CIBERSORT analysis also showed that IGFLR1 expression was significantly positively associated with the immune cells infiltration in cholangiocarcinoma (CHOL), showing the highest correlation coefficient. Among the immune cell population, the infiltration level of CD4+ T cells and dendritic cells was consistent with the varying trend in the expression of IGFLR1 (Fig. 5).
Mechanism of IGFLR1
To identify potential pathways associated with IGFLR1, we explored the mechanism of IGFLR1. We found that in six different cancers, there was a direct interaction between IGFLR1 and GATA3 (Pathway commons), among which CHOL had the strongest correlation (P < 0.05) (Table S11). Subsequently, by co-expression analysis, it was found that in multiple cancers, related genes in the interferon receptor (INFR) pathway were co-expressed with IGFLR1 at a higher level (Pearson, P < 0.05) (Fig. S1). This suggested that IGFLR1 might affect disease progression by participating in related steps of INFR pathway.
Discussion
Accurate stratification of cancer patients is key to predict the prognosis and provide personalized treatment plans. However, the available stratification methods lack accuracy. Increasing number of studies have shown that immune cell infiltration characteristics in the tumor microenvironment are closely related to patient prognosis, and stratification tools based on immune cell infiltration characteristics are currently being evaluated by researchers [27]. Many of these tools have been shown to have a good diagnostic value for guiding treatment strategies [28, 29].
IGFLR1 is encoded by the IGFLR1 gene, a conserved gene in mammals, also known as TMEM149. IGFLR1 is a type 1α transmembrane protein that was found to be expressed in T cells and monocytes in the mouse with psoriasis in 2011 [30]. It was subsequently confirmed that IGFLR1 was related to skin inflammatory signaling [31]. And IGFLR1 was considered a new member of the TNFR family due to its similar structure to the nerve growth factor receptor (NGFR) [30, 32, 33], which monoclonal antibodies against NGFR have been shown to regulate chemotherapy sensitivity [34]. In addition, a study reported that the partially folded region of IGFLR1 was highly similar with the death domain, which suggested that IGFLR1 might be associated with apoptosis [30]. In CRC, researchers confirmed IGFLR1 was highly expressed in CXCL13+BHLHE40+TH1-like cells, CD8+Tex cells and Treg cells [9, 10], and IGFLR1 was the top 9 mutational genes that mutated in >10% of the CRC cell lines, which were confirmed by Sanger sequencing [35]. Those suggested IGFLR1 plays an important role in CRC and its immune responsibility.
In this work, we identified the expression of IGFLR1 and the relationship between IGFLR1 and other factors. Our study found IGFLR1 significant different expressed in the cancer tissue and normal tissue, which suggested it may have a role in the occurrence and development of cancer. This real-world data is highly accurate and reproducible. Regarding the association between IGFLR1 and microsatellite status, our study showed that IGFLR1 expression in stage II and III CRC was independent of microsatellite status. However, in Zemin Zhang’s research, they included four patients with MSI-H and eight patients with MSS and found that CXCL13+BHLHE40+ TH1-like cells were highly abundant in MSI-H CRC patients, and IGFLR1 was highly expressed on the surface of CXCL13+BHLHE40+ TH1 cells [9, 10], which suggested that IGFLR1 might be associated with microsatellite status. However, the above data was indirect evidence and which needs to be further investigated for more causal link.
Subsequently, we found that IGFLR1 had a good correlation with FOXP3, CD8 and gender. Further, we utilized CIBERSORT, TIMER, and EPIC and found the expression of IGFLR1 was associated with immune cell infiltration. TIMER, EPIC, and CIBERSORT are three kinds of immune infiltration scoring methods. TIMER and EPIC contained similar types of immune cells, and their results were highly consistent; while CIBERSORT refined the types of immune cells, which was more helpful in the interpretation of immune mechanisms. So, the results from CIBERSHOT might differ from the other two methods. The above results demonstrated a correlation between IGFLR1 and immune cell infiltration. Also, in previous studies, Zemin Zhang’s team used single-cell RNA sequencing to analyze 11,138 T cells from 12 CRC patients, and found that IGFLR1 expression was higher in CXCL13+BHLHE40+ TH1-like cells, CD8+Tex cells, and Treg cells in CRC patients [9]. Elisa Donnard’s team did a mutational analysis of genes coding for cell surface proteins in CRC cell lines reveal novel altered pathways,which found IGFLR1 was the top 9 mutational genes that mutated in >10% of the CRC cell lines [35]. And in Jingwei Zhang’s team, they found IGFLR1 was associated with the infiltration of CD8+ T cells and myeloid suppressor cells (MDSCs) [36]. CXCL13+BHLHE40+ TH1 cell plays an important role in regulating immune microenvironment and immune response in MSI-H CRC [9]. Treg is a suppressive immune cell which associated with poor prognosis, and FOXP3 is a known biomarker associated with Tregs [37, 38]. MDSCs also exist as inhibitory components in the tumor immune microenvironment and have ability to suppress immune cell responses [39]. CD8+Tex is a class of cells that have lost T cell function. They express various inhibitory receptors and have a dysregulated metabolism and a poor memory recall response characteristics [40]. All the above data suggested that IGFLR1 played an important role in modulating the tumor immune microenvironment.
In terms of prognosis, we found that IGFLR1 had a significant impact on the prognosis of patients with stage II and III CRC. Furthermore, a molecular stratification model including IGFLR1, FOXP3, PD-1, and CD4 was shown to have a good ability for prognostic stratification. Also, we screened IGFLR1-related differential genes in bioinformatics database and these differential genes were found to be significantly associated with immune cell infiltration and patient prognosis, which could be used for risk stratification. Jingwei Zhang’s team also found similar results [36]. The above findings indicated that IGFLR1 could be a reliable prognostic stratification marker, which could help to further guide tumor prognosis and precision therapy. However, the model needs to be evaluated in a larger number of patients to validate the current findings.
In terms of the mechanism of IGFLR1, we found that there were common pathways between IGFLR1 and GATA3 in various cancers, and related proteins in the INFR pathway had a high co-expression with IGFLR1. GATA3 is an immune-related gene, which not just regulates the differentiation of Th2 cells, but also plays an important role in a variety of immune cells [41,42,43]. Our study verified that IGFLR1 might interact with GATA3 to play an important role in multiple aspects of anti-tumor immunity, which deserves further investigation. IFNs play important roles in proliferation, immune regulation and anti-tumor mechnisms. By regulating the IFNR signaling pathway, the expression of a variety of proliferation-related genes can be modulated, thereby affecting tumor proliferation, metastasis, apoptosis, cell cycle, angiogenesis, microenvironment stability, etc. These pathways are known to regulate immune response and drug resistance of tumors [44]. Our results suggested that IGFLR1 may be involved in the INFR pathway, which provided a good idea for further research.
Previous studies have shown that among the four ligands of IGFLR1 (IGFL1-4), IGFL1 and IGFL3 have high affinity for IGFLR1 [9, 10, 30]. IGFL1 can specifically bind to IGFLR1, which then regulates the inflammatory response and apoptosis [30]. The binding of IGFL3 and IGFLR1 on CD4+ T cells can enhance the expression of CD25 and INF-γ and the secretion of INF-γ is positively correlated with the IGFLR1 in T cells. This process can be specifically blocked by IGFLR1 antibody [9, 10]. These findings suggested that IGFLR1 might play a role by regulating the INFR pathway after binding to IGFL3. Therefore, in-depth studies on IGFLR1-related pathways may enable the identification of new immunosuppressive targets and improve the efficacy of existing immunosuppressants. The above findings suggest that as a novel molecule, IGFLR1 has a great potential as a therapeutic target in oncology and hence deserves greater attention [9, 10, 30, 36].
In summary, in this study, we focused on a new biomarker called IGFLR1. Our results based on a sufficient number of pathological sections, which is an important step in moving IGFLR1 from theoretical exploration to practical application. Our results support further investigations of IGFLR1 in solid tumors and provide some support for subsequent works. However, there were some limitations in this study. Firstly, we only included patients with stage II and III CRC. Secondly, our research did not reveal the precise mechanism of IGFLR1 in CRC. Finally, as a retrospective analysis, the present study is less convincing than a prospective analysis. Therefore, we need further studies with a better design and more in-depth mechanistic investigation to enable the clinical application of IGFLR1 as a candidate target in cancer.
Conclusion
IGFLR1 has emerged as a new molecule related to prognosis and immune cell infiltration in CRC. The prognostic model based on IGFLR1 and IGFLR1-related genes showed a good ability to predict patient prognosis and stratify patients into low- and high-risk groups. In addition, IGFLR1 plays an important role in GATA3, IINFR, and other pathways, suggesting it deserves further study.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Dekker, E., Tanis, P. J., Vleugels, J. L. A., et al. (2019). Colorectal cancer. Lancet, 394, 1467–80.
Schreuders, E. H., Ruco, A., Rabeneck, L., et al. (2015). Colorectal cancer screening: A global overview of existing programmes. Gut, 64, 1637–49.
Wang, W., Kandimalla, R., Huang, H., et al. (2019). Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Seminars in Cancer Biology, 55, 37–52.
Galon, J., Angell, H. K., Bedognetti, D., et al. (2013). The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity, 39, 11–26.
Van den Eynde, M., Mlecnik, B., Bindea, G., et al. (2018). The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell, 34, 1012–26.e3.
Almquist, D. R., Ahn, D. H., & Bekaii-Saab, T. S. (2020). The role of immune checkpoint inhibitors in colorectal adenocarcinoma. BioDrugs, 34, 349–62.
Du, F., & Liu, Y. (2022). Predictive molecular markers for the treatment with immune checkpoint inhibitors in colorectal cancer. Journal of Clinical Laboratory Analysis, 36, e24141.
Lizardo, D. Y., Kuang, C., Hao, S., et al. (2020). Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer, 1874, 188447.
Zhang, L., Yu, X., Zheng, L., et al. (2018). Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature, 564, 268–72.
Ren, X., & Zhang, Z. (2019). Understanding tumor-infiltrating lymphocytes by single cell RNA sequencing. Advances in Immunology in China - Part A, 144, 217–45.
Liu, H., Li, Y., Li, J., et al. (2018). H3K4me3 and Wdr82 are associated with tumor progression and a favorable prognosis in human colorectal cancer. Oncology Letters, 16, 2125–34.
Orekoya, A. A., Abdus-Salam, A. A., Oyesegun, A. R., et al. (2023). EGFR and HER2 expression in cervical cancer patients in Ibadan, Nigeria. Ecancermedicalscience, 17, 1607.
Remmele, W., & Stegner, H. E. (1987). Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe,8, 138–40.
Shengmin, Chen, Guoping, Wang, Zhiren, Lin, et al. (2021). Expression of PKCι/YAP1 in cervical cancer and its clinical significance. Cancer Research on Prevention and Treatment, 48, 970–3.
Jiali, Liu, Beibei, Xuan, Quan, Quan, et al. (2022). Significance of transcription factor LMO3 in evaluating prognosis of patients with endometrial carcinoma. Journal of Army Medical University, 44, 1054–60.
DeLong, E. R., DeLong, D. M., & Clarke-Pearson, D. L. (1988). Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics, 44, 837–45.
Laska, E., Meisner, M., & Wanderling, J. (2012). A maximally selected test of symmetry about zero. Statistics in Medicine, 31, 3178–91.
Ritchie, M. E., Phipson, B., Wu, D., et al. (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research, 43, e47.
Yoshihara, K., Shahmoradgoli, M., Martínez, E., et al. (2013). Inferring tumour purity and stromal and immune cell admixture from expression data. Nature Communications, 4, 2612.
Langfelder, P., & Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics, 9, 559.
Friedman, J., Hastie, T., & Tibshirani, R. (2010). Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software, 33, 1–22.
Heagerty, P. J., & Zheng, Y. (2005). Survival model predictive accuracy and ROC curves. Biometrics, 61, 92–105.
Newman, A. M., Liu, C. L., Green, M. R., et al. (2015). Robust enumeration of cell subsets from tissue expression profiles. Nature Methods, 12, 453–7.
Li, T., Fan, J., Wang, B., et al. (2017). TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Reseasrch, 77, e108–e10.
Racle J, de Jonge K, Baumgaertner P, et al. (2017). Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife, 6. https://doi.org/10.7554/eLife.26476
Chen, B., Khodadoust, M. S., Liu, C. L., et al. (2018). Profiling tumor infiltrating immune cells with CIBERSORT. Methods in Molecular Biology, 1711, 243–59.
Lin, Y., Pan, X., Zhao, L., et al. (2021). Immune cell infiltration signatures identified molecular subtypes and underlying mechanisms in gastric cancer. npj Genomic Medicine, 6, 83.
Zhu, X., Tian, X., Ji, L., et al. (2021). A tumor microenvironment-specific gene expression signature predicts chemotherapy resistance in colorectal cancer patients. npj Precision Oncology, 5, 7.
Fan, J., Liu, Y., Cai, X., et al. (2020). A Novel Prognostic Model Incorporating carcinoembryonic antigen in 3-week or longer postoperative period for stage III colon cancer: A multicenter retrospective study. Frontiers in Oncology, 10, 566784.
Lobito, A. A., Ramani, S. R., Tom, I., et al. (2011). Murine insulin growth factor-like (IGFL) and human IGFL1 proteins are induced in inflammatory skin conditions and bind to a novel tumor necrosis factor receptor family member, IGFLR1. Journal of Biological Chemistry, 286, 18969–81.
Konger RL, Derr-Yellin E, Zimmers TA, et al. (2021). Epidermal PPARγ is a key homeostatic regulator of cutaneous inflammation and barrier function in mouse skin. International journal of molecular sciences, 22. https://doi.org/10.3390/ijms22168634
Bodmer, J. L., Schneider, P., & Tschopp, J. (2002). The molecular architecture of the TNF superfamily. Trends in Biochemical Sciences, 27, 19–26.
Söding, J. (2005). Protein homology detection by HMM-HMM comparison. Bioinformatics, 21, 951–60.
Garcia-Garcia, R. M., Arias-Alvarez, M., Sanchez-Rodriguez, A., et al. (2020). Role of nerve growth factor in the reproductive physiology of female rabbits: A review. Theriogenology, 150, 321–8.
Donnard, E., Asprino, P. F., Correa, B. R., et al. (2014). Mutational analysis of genes coding for cell surface proteins in colorectal cancer cell lines reveal novel altered pathways, druggable mutations and mutated epitopes for targeted therapy. Oncotarget, 5, 9199–213.
Song, W., Shao, Y., He, X., et al. (2020). IGFLR1 as a novel prognostic biomarker in clear cell renal cell cancer correlating with immune infiltrates. Frontiers in Molecular Biosciences, 7, 565173.
Martin, F., Ladoire, S., Mignot, G., et al. (2010). Human FOXP3 and cancer. Oncogene, 29, 4121–9.
Togashi, Y., Shitara, K., & Nishikawa, H. (2019). Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nature Reviews Clinical Oncology, 16, 356–71.
Hegde, S., Leader, A. M., & Merad, M. (2021). MDSC: Markers, development, states, and unaddressed complexity. Immunity, 54, 875–84.
Kurachi, M. (2019). CD8(+) T cell exhaustion. Seminars in Immunopathology, 41, 327–37.
Wan, Y. Y. (2014). GATA3: a master of many trades in immune regulation. Trends in Immunology, 35, 233–42.
Rothenberg, E. V. (2013). GATA-3 locks the door to the B-cell option. Blood, 121, 1673–4.
Mjösberg, J., Bernink, J., Golebski, K., et al. (2012). The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity, 37, 649–59.
Benci, J. L., Xu, B., Qiu, Y., et al. (2016). Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell, 167, 1540–54.e12.
Funding
The current work was supported by the Haiyan Foundation of Harbin Medical University Cancer Hospital (JJZD2022-17), Nn10 project of Harbin medical university cancer hospital (no.04000079), Beijiing Yanchuang Foundation (ZLKY-20211129-02) and the Medical Wisdom Research Fund by the Heilongjiang Sunshine Health Foundation (H21L0802). Author Yanlong Liu has received research supports from funding JJZD2022-17, ZLKY-20211129-02, and H21L0802. Author Jinxue Tong has received research supports from funding no.04000079.
Author information
Authors and Affiliations
Contributions
Yanlong Liu, Jinxue Tong, and Qiuju Zhang made a substantial contribution to the concept or design of the work. Fenqi Du, Xinhao Han, Junnan Guo, Wenjie Song, Yixiu Xia, Xinyu Yue, Da Yang, and Ran Jin did the acquisition, analysis, or interpretation of data. Ran Jin, Fenqi Du, and Junnan Guo drafted the article. Yanlong Liu, Qiuju Zhang, and Jinxue Tong revised it critically for important intellectual content and approved the version to be published.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The study was approved by the ethics committee of Harbin Medical University Cancer Hospital (ethical approval number: KY2022-11). All patients included in this study were signed the “Informed Consent for Secondary Use of Biological Specimens.” This was a power of attorney document, which allowed us to use and publish their clinical and pathological information as well as biological specimens in a way that protected their privacy.
Consent for Publication
None
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, R., Du, F., Han, X. et al. Prognostic Value of Insulin Growth Factor-Like Receptor 1 (IGFLR1) in Stage II and III Colorectal Cancer and Its Association with Immune Cell Infiltration. Appl Biochem Biotechnol (2024). https://doi.org/10.1007/s12010-024-05006-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-024-05006-1