Abstract
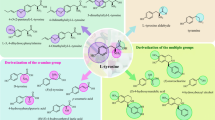
L-Alanyl-L-tyrosine (L-Ala-Tyr) is a dipeptide formed by the condensation of L-alanine methyl ester and L-tyrosine. After entering the body, it can be rapidly broken down to release tyrosine. In this study, L-Ala-Tyr was successfully prepared by using α-ester acyltransferase as biocatalyst and alanine methyl ester (L-Ala-OMe) and tyrosine (L-Tyr) as acyl donor and nucleophile, respectively. The dipeptide yield was increased from 15 to 50% by optimizing the conditions: boric acid-borax (0.2 mol/L), 30°C, pH 9.5, 2:1 acyl donor to nucleophile ratio, DES (ChCl/urea), and 15%(v/v) water content. The catalytic product is then isolated and purified. The structure of the product was identified by high-performance liquid chromatography, mass spectrometry, proton nuclear magnetic resonance, and carbon spectroscopy. Its biological activity was preliminarily determined by the B16-F10 mouse melanoma cell model. The results showed that the purity of L-Ala-Tyr prepared by the separation and purification method of this study was 96.8%, and the mass spectrometry and nuclear magnetic resonance spectroscopy showed that the structure of the peptide was consistent with the expected structure. In addition, the preliminary physiological activity identification results show that L-Ala-Tyr has no toxic effect on cells in the concentration range of 100–800 μmol·L−1, and at the optimal concentration, compared with the positive control 8-methoxypsoralen, it can promote the production of melanin.







Similar content being viewed by others
Data Availability
All further relevant data generated or analyzed during this study are included in this published article and its supplementary files.
References
Abbott, A. P., Capper, G., Davies, D. L., Rasheed, R. K., & Tambyrajah, V. (2003). Novel solvent properties of choline chloride/urea mixtures. Chemical Communications, (1), 70–71. https://doi.org/10.1039/B210714G.
Abe, I., Hara, S., & Yokozeki, K. (2011). Gene cloning and characterization of alpha-amino acid ester acyl transferase in Empedobacter brevis ATCC14234 and Sphingobacterium siyangensis AJ2458. Bioscience, Biotechnology, and Biochemistry, 75(11), 2087–2092. https://doi.org/10.1271/bbb.110181
Carta, R., & Tola, G. (1996). Solubilities of l-cystine, l-tyrosine, l-leucine, and glycine in aqueous solutions at various pHs and NaCl concentrations. Journal of Chemical & Engineering Data, 41(3), 414–417. https://doi.org/10.1021/je9501853
Chalamaiah, M., Yu, W., & Wu, J. (2018). Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chemistry, 245, 205–222. https://doi.org/10.1016/j.foodchem.2017.10.087
Chung, H., Jung, H., Lee, J. H., Oh, H. Y., Kim, O. B., Han, I. O., & Oh, E. S. (2014). Keratinocyte-derived laminin-332 protein promotes melanin synthesis via regulation of tyrosine uptake. The Journal of Biological Chemistry, 289(31), 21751–21759. https://doi.org/10.1074/jbc.M113.541177
Daabees, T. T., & Stegink, L. D. (1978). L-Alanyl-L-tyrosine as a tyrosine source during intravenous nutrition of the rat. The Journal of Nutrition, 108(7), 1104–1113. https://doi.org/10.1093/jn/108.7.1104
Guajardo, N., & Domínguez de María, P. (2019). Continuous biocatalysis in environmentally-friendly media: A triple synergy for future sustainable processes. ChemCatChem, 11(14), 3128–3137. https://doi.org/10.1002/cctc.201900773
Huo, T., Ma, J. J., Shen, N., Jia, Q. Y., & Liu, Q. F. (2014). Chemical synthesis of L-alanyl-L-tyrosine dipeptide. Chemical Technology and Development, 43(02), 16–17. https://doi.org/10.3969/j.issn.1671-9905.2014.02.005
Lee, Y., & Hyun, C. G. (2022). Mechanistic insights into the ameliorating effect of melanogenesis of psoralen derivatives in B16F10 melanoma cells. Molecules, 27(9). https://doi.org/10.3390/molecules27092613
LeWitt, T. M., & Kundu, R. V. (2021). Vitiligo. JAMA Dermatology, 157(9), 1136–1136. https://doi.org/10.1001/jamadermatol.2021.1688
Li, Y. M., Gao, J. Q., Pei, X. Z., Du, C., Fan, C., Yuan, W. J., & Bai, F. W. (2019). Production of L-alanyl-L-glutamine by immobilized Pichia pastoris GS115 expressing alpha-amino acid ester acyltransferase. Microbial Cell Factories, 18(1), 27. https://doi.org/10.1186/s12934-019-1077-1
Liu, P. F., Lu, Q. M., Hu, X. Q., Hou, X. W., & Zhang, H. B. (2018). Expression, purification, characterization and application of α-amino acid ester acyltransferase from recombinant Escherichia coli. Chinese Journal of Biotechnology, 34(7), 9. https://doi.org/10.13345/j.cjb.180017
Maher, T. J., Kiritsy, P. J., Moya-Huff, F. A., Casacci, F., De Marchi, F., & Wurtman, R. J. (1990). Use of parenteral dipeptides to increase serum tyrosine levels and to enhance catecholamine-mediated neurotransmission. Journal of Pharmaceutical Sciences, 79(8), 685–687. https://doi.org/10.1002/jps.2600790807
Mahmoud, D. B., ElMeshad, A. N., Fadel, M., Tawfik, A., & Ramez, S. A. (2022). Photodynamic therapy fortified with topical oleyl alcohol-based transethosomal 8-methoxypsoralen for ameliorating vitiligo: Optimization and clinical study. International Journal of Pharmaceutics, 614, 121459. https://doi.org/10.1016/j.ijpharm.2022.121459
Najem, A., Wouters, J., Krayem, M., Rambow, F., Sabbah, M., Sales, F., Awada, A., Aerts, S., Journe, F., Marine, J. C., & Ghanem, G. E. (2021). Tyrosine-dependent phenotype switching occurs early in many primary melanoma cultures limiting their translational value. Frontiers in Oncology, 11, 780654. https://doi.org/10.3389/fonc.2021.780654
Santos, S., Torcato, I., & Castanho, M. A. (2012). Biomedical applications of dipeptides and tripeptides. Biopolymers, 98(4), 288–293. https://doi.org/10.1002/bip.22067
Sheldon, R. A., & Woodley, J. M. (2018). Role of biocatalysis in sustainable Chemistry. Chemical Reviews, 118(2), 801–838. https://doi.org/10.1021/acs.chemrev.7b00203
Slominski, A., Moellmann, G., Kuklinska, E., Bomirski, A., & Pawelek, J. (1988). Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. Journal of Cell Science, 89(3), 287–296. https://doi.org/10.1242/jcs.89.3.287
Slominski, A., Zmijewski, M. A., & Pawelek, J. (2012). L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell & Melanoma Research, 25(1), 14–27. https://doi.org/10.1111/j.1755-148X.2011.00898.x
Tan, J. N., & Dou, Y. (2020). Deep eutectic solvents for biocatalytic transformations: Focused lipase-catalyzed organic reactions. Applied Microbiology and Biotechnology, 104(4), 1481–1496. https://doi.org/10.1007/s00253-019-10342-y
Wang, M., Qi, W., Yu, Q., Su, R., & He, Z. (2011). Kinetically controlled enzymatic synthesis of dipeptide precursor of L-alanyl-L-glutamine. Biotechnology and Applied Biochemistry, 58(6), 449–455. https://doi.org/10.1002/bab.55
Wang, T., Zhang, Y.-R., Liu, X.-H., Ge, S., & Zhu, Y.-S. (2019). Strategy for the biosynthesis of short oligopeptides: Green and sustainable chemistry. Biomolecules, 9(11). https://doi.org/10.3390/biom9110733
Wang, X., Chen, Y., Lan, B., Wang, Y., Lin, W., Jiang, X., Ye, J., Shang, B., Feng, C., Liu, J., Zhai, J., Xu, M., Li, Q., Lin, L., Hu, M., Zheng, F., Chen, L., Shao, C., Wang, Y., & Shi, Y. (2022). Heterogeneity of tyrosine-based melanin anabolism regulates pulmonary and cerebral organotropic colonization microenvironment of melanoma cells. Theranostics, 12(5), 2063–2079. https://doi.org/10.7150/thno.69198
Xiong, J., Cao, S. L., Zong, M. H., Lou, W. Y., & Wu, X. L. (2020). Biosynthesis of alanyl-histidine dipeptide catalyzed by papain immobilized on magnetic nanocrystalline cellulose in deep eutectic solvents. Applied Biochemistry and Biotechnology, 192(2), 573–584. https://doi.org/10.1007/s12010-020-03345-3
Yokozeki, K., & Hara, S. (2005). A novel and efficient enzymatic method for the production of peptides from unprotected starting materials. Journal of Biotechnology, 115(2), 211–220. https://doi.org/10.1016/j.jbiotec.2004.07.017
Funding
This study was supported by the Anhui Provincial Natural Science Foundation (grant no. 2108085MC120) and Anhui Province Key Research and Development Plan Project 2023s07020013.
Author information
Authors and Affiliations
Contributions
YN Fan: software, validation, formal analysis, investigation, writing (original draft), and visualization. JA Wei: software and formal analysis. ZW Li: software. JW Yang: methodology. HB Zhang: conceptualization and supervision. XQ Hu: conceptualization, formal analysis, resources, data curation, writing (review and editing), visualization, and funding acquisition.
Corresponding authors
Ethics declarations
Ethical approval
This study does not contain any studies with human participants or animals performed.
Conflict of interest
The authors declare competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• This study provides a method to synthesize dipeptide by using α-ester acyltransferase in DES.
• Through process optimization, the conversion rate of the substrate was improved.
• The physiological activity of Ala-Tyr was proved by the melanocyte experiment.
Supplementary Information
ESM 1
(DOCX 183 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Wei, J., Li, Z. et al. Biosynthesis, Characterization, and Bioactivity of L-Alanyl-L-tyrosine in Promoting Melanin Synthesis. Appl Biochem Biotechnol (2023). https://doi.org/10.1007/s12010-023-04713-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04713-5