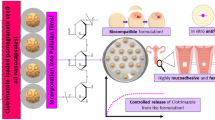
Abstract
Currently, the high incidence of fungal infections among females has resulted in outstanding problems. Candida species is related with multidrug resistance and destitute clinical consequences. Chitosan-albumin derivatives with more stability exhibit innate antifungal and antibacterial effects that boost the activity of the drug without inflammatory impact. The stability and sustained release of Fluconazole in mucosal tissues can be ensured by encapsulating in protein/polysaccharide nanocomposites. Thus, we developed chitosan-albumin nanocomposite (CS-A) loaded with Fluconazole (Flu) antifungals against vaginal candidiasis. Various ratios of CS/Flu (1:1, 1:2, 2:1) were prepared. Thereafter, the CS-A-Flu nanocomposites were qualified and quantified using FT-IR, DLS, TEM, and SEM analytical devices, and the size range from 60 to 100 nm in diameter was attained for the synthesized nanocarriers. Afterward, the antifungal activity, biofilm reduction potency, and cell viability assay were performed for biomedical evaluation of formulations. The minimum inhibitory concentration) and minimum fungicidal concentration on Candida albicans were attained at 125 ng/μL and 150 ng/μL after treatment with a 1:2 (CS/Flu) ratio of CS-A-Flu. The biofilm reduction assay indicated that biofilm formation was between 0.05 and 0.1% for CS-A-Flu at all ratios. The MTT assay also exhibited excellent biocompatibility for samples, about 7 to 14% toxicity on human HGF normal cells. These data have indicated that CS-A-Flu would be a promising candidate against Candida albicans.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
MacAlpine, J., Robbins, N., & Cowen, L. E. (2022). Bacterial-fungal interactions and their impact on microbial pathogenesis. Molecular Ecology. https://doi.org/10.1111/mec.16411
Carvalho, G. C., et al. (2021). Prevalence of vulvovaginal candidiasis in Brazil: A systematic review. Medical Mycology, 59(10), 946–957.
Palmeira-de-Oliveira, R., et al. (2022). Women’s preferences and acceptance for different drug delivery routes and products. Advanced Drug Delivery Reviews, 114133.
Kumari, R., Sunil, D., & Ningthoujam, R. S. (2020). Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. Journal of Controlled Release, 319, 135–156.
Naderlou, E., et al. (2020). Enhanced sensitivity and efficiency of detection of Staphylococcus aureus based on modified magnetic nanoparticles by photometric systems. Artificial Cells, Nanomedicine, and Biotechnology, 48(1), 810–817.
Narmani, A., & Jafari, S. M. (2021). Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydrate Polymers, 272, 118464.
Chen, Y., et al. (2018). Evaluation of the PEG density in the PEGylated chitosan nanoparticles as a drug carrier for curcumin and mitoxantrone. Nanomaterials, 8(7), 486.
Niu, S., et al. (2019). A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. Journal of Nanobiotechnology, 17(1), 1–18.
Khakinahad, Y., et al. (2022). Margetuximab conjugated-PEG-PAMAM G4 nano-complex: A smart nano-device for suppression of breast cancer. Biomedical Engineering Letters, 12(3), 317.
Wang, Y., et al. (2016). Nanoparticles of chitosan conjugated to organo-ruthenium complexes. Inorganic Chemistry Frontiers, 3(8), 1058–1064.
Kong, M., et al. (2010). Antimicrobial properties of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology, 144(1), 51–63.
Caraceni, P., et al. (2013). Clinical indications for the albumin use: Still a controversial issue. European Journal of Internal Medicine, 24(8), 721–728.
Kianfar, E. (2021). Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. Journal of Nanobiotechnology, 19(1), 159.
Ogawa, M., et al. (2014). Plasma antithrombin levels correlate with albumin and total protein in gestational hypertension and preeclampsia. Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health, 4(2), 174–177.
Parodi, A., et al. (2019). Albumin nanovectors in cancer therapy and imaging. Biomolecules, 9(6), 218.
Wong, C. Y., Martinez, J., & Dass, C. R. (2016). Oral delivery of insulin for treatment of diabetes: Status quo, challenges and opportunities. Journal of Pharmacy and Pharmacology, 68(9), 1093–1108.
Walke, S., et al. (2015). Fabrication of chitosan microspheres using vanillin/TPP dual crosslinkers for protein antigens encapsulation. Carbohydrate Polymers, 128, 188–198.
El-Housiny, S., et al. (2018). Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Delivery, 25(1), 78–90.
Rençber, S., et al. (2019). Formulation and evaluation of fluconazole loaded oral strips for local treatment of oral candidiasis. Journal of Drug Delivery Science and Technology, 49, 615–621.
Chayachinda, C., et al. (2022). Effect of intravaginal gentian violet for acute vaginal candidiasis treated with a single dose oral fluconazole: A randomised controlled trial. Journal of Obstetrics and Gynaecology, 42(6), 2190.
Kelidari, H. R., et al. (2017). Improved yeast delivery of fluconazole with a nanostructured lipid carrier system. Biomedicine & Pharmacotherapy, 89, 83–88.
Faramarzi, N., et al. (2020). Synthesis and in vitro Evaluation of tamoxifen-loaded gelatin as effective nanocomplex in drug delivery systems. International Journal of Nanoscience, 19(5), 2050002.
Cantón, E., et al. (2003). Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagnostic Microbiology and Infectious Disease, 45(3), 203–206.
Fotouhi, P., et al. (2021). Surface modified and rituximab functionalized PAMAM G4 nanoparticle for targeted imatinib delivery to leukemia cells: In vitro studies. Process Biochemistry, 111, 221–229.
Bandara, S., et al. (2018). Synthesis and characterization of Zinc/Chitosan-Folic acid complex. Heliyon, 4(8), e00737.
Kaur, K., et al. (2015). Wheat germ agglutinin anchored chitosan microspheres of reduced brominated derivative of noscapine ameliorated acute inflammation in experimental colitis. Colloids and Surfaces B: Biointerfaces, 132, 225–235.
Tan, W., et al. (2018). Novel cationic chitosan derivative bearing 1, 2, 3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydrate Polymers, 182, 180–187.
El Rabey, H. A., et al. (2019). Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. International Journal of Biological Macromolecules, 141, 511–516.
Yousefi, M., Narmani, A., & Jafari, S. M. (2020). Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Advances in Colloid And Interface Science, 278, 102125.
Rezvani, M., et al. (2018). Synthesis and in vitro study of modified chitosan-polycaprolactam nanocomplex as delivery system. International Journal of Biological Macromolecules, 113, 1287–1293.
Narmani, A., et al. (2018). Targeting delivery of oxaliplatin with smart PEG-modified PAMAM G4 to colorectal cell line: In vitro studies. Process Biochemistry, 69, 178–187.
Golafzani, F. N., et al. (2022). Delivery of miRNA-126 through folic acid-targeted biocompatible polymeric nanoparticles for effective lung cancer therapy. Journal of Bioactive and Compatible Polymers, 37(3), 168–188.
Narmani, A., et al. (2020). Breast tumor targeting with PAMAM-PEG-5FU-99mTc as a new therapeutic nanocomplex: In in-vitro and in-vivo studies. Biomedical Microdevices, 22(2), 1–13.
Fitaihi, R. A., et al. (2018). Role of chitosan on controlling the characteristics and antifungal activity of bioadhesive fluconazole vaginal tablets. Saudi Pharmaceutical Journal, 26(2), 151–161.
Tiboni, M., et al. (2021). 3D printed clotrimazole intravaginal ring for the treatment of recurrent vaginal candidiasis. International Journal of Pharmaceutics, 596, 120290.
Meng, Q., et al. (2021). An overview of chitosan and its application in infectious diseases. Drug Delivery and Translational Research, 11, 1340–1351.
Li, L., et al. (2022). Amphiphilic nano-delivery system based on modified-chitosan and ovalbumin: Delivery and stability in simulated digestion. Carbohydrate Polymers, 294, 119779.
Calvo, N. L., et al. (2019). Chitosan-hydroxypropyl methylcellulose tioconazole films: A promising alternative dosage form for the treatment of vaginal candidiasis. International Journal of Pharmaceutics, 556, 181–191.
Silva-Dias, A., et al. (2014). Anti-biofilm activity of low-molecular weight chitosan hydrogel against Candida species. Medical Microbiology and Immunology, 203, 25–33.
Jabali, M. K., et al. (2022). Design of a pDNA nanocarrier with ascorbic acid modified chitosan coated on superparamagnetic iron oxide nanoparticles for gene delivery. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 632, 127743.
Abdellatif, M. M., et al. (2020). Formulation and characterization of sertaconazole nitrate mucoadhesive liposomes for vaginal candidiasis. International Journal of Nanomedicine, 15, 4079.
Dara, P. K., et al. (2021). Biocompatibility and histopathological evaluation of chitosan nanoparticles grafted fish gelatin bio-nanocomposite membranes in rats. Iranian Polymer Journal, 30(9), 953–964.
Author information
Authors and Affiliations
Contributions
MHA: study concept and design, acquisition of data, drafting of the manuscript. JM: critical revision of the manuscript for important intellectual content. SK: statistical analysis, administrative, technical and material support, study supervision, analysis and interpretation of data.
Corresponding author
Ethics declarations
Consent to Participate
The authors declare that they have consent to participate
Consent for Publication
The authors declare that they have consent to publish
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hatamiazar, M., Mohammadnejad, J. & Khaleghi, S. Chitosan-Albumin Nanocomposite as a Promising Nanocarrier for Efficient Delivery of Fluconazole Against Vaginal Candidiasis. Appl Biochem Biotechnol 196, 701–716 (2024). https://doi.org/10.1007/s12010-023-04492-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04492-z