Abstract
Background: Colorectal cancer (CRC) is a common malignancy of the gastrointestinal tract with high incidence and mortality. Exosomal circular RNA (circRNA) has been shown to be associated with the malignant progression of cancers, including CRC. Circ_0005100 (named as circ_FMN2) has been shown to promote CRC cell proliferation and migration. However, whether exosomal circ_FMN2 participated in CRC progression remains unclear. Methods: Exosomes were isolated from the serum of CRC patients and then identified using transmission electron microscope. Western blot assay was used to test the protein levels of exosome markers, proliferation-related marker, metastasis-related markers and musashi-1 (MSI1). The expression levels of circ_FMN2, microRNA (miR)-338-3p and MSI1 were detected by qPCR. Flow cytometry, colony formation assay, MTT assay, and transwell assay were employed to measure cell cycle, apoptosis, colony formation ability, viability, migration and invasion. Dual-luciferase reporter assay was performed to assess the interaction between miR-338-3p and circ_FMN2 or MSI1. BALB/c nude mice was used to conduct animal experiments. Results: Circ_FMN2 was overexpressed in the exosomes of CRC patient’s serums and CRC cells. Overexpressed exosomal circ_FMN2 could promote CRC cell proliferation, metastasis, and suppress apoptosis. Circ_FMN2 acted as miR-338-3p sponge. MiR-338-3p overexpression reversed the promotion effect of circ_FMN2 on CRC progression. MSI1 was found to be a target of miR-338-3p, and its overexpression revoked the inhibitory effect of miR-338-3p on CRC progression. Furthermore, exosomal circ_FMN2 overexpression also could facilitate CRC tumor growth in vivo. Conclusion: Exosomal circ_FMN2 accelerated CRC progression through miR-338-3p/MSI1 axis, revealing that exosomal circ_FMN2 might be a target for CRC treatment.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
27 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12010-023-04722-4
References
Brody, H. (2015). Colorectal cancer. Nature, 521, S1.
Nasseri, Y., & Langenfeld, S. J. (2017). Imaging for Colorectal Cancer. Surgical Clinics Of North America, 97, 503–513.
Cho, Y. A., Lee, J., Oh, J. H., Chang, H. J., Sohn, D. K., Shin, A., et al. (2019). Genetic risk score, combined lifestyle factors and risk of Colorectal Cancer. Cancer Res Treat, 51, 1033–1040.
Carr, P. R., Weigl, K., Jansen, L., Walter, V., Erben, V., Chang-Claude, J., et al. (2018). Healthy lifestyle factors Associated with Lower Risk of Colorectal Cancer irrespective of genetic risk. Gastroenterology, 155, 1805–1815e1805.
Buccafusca, G., Proserpio, I., Tralongo, A. C., Rametta Giuliano, S., & Tralongo, P. (2019). Early colorectal cancer: Diagnosis, treatment and survivorship care. Critical Reviews In Oncology Hematology, 136, 20–30.
Jin, M., & Frankel, W. L. (2018). Lymph Node Metastasis in Colorectal Cancer. Surgical Oncology Clinics Of North America, 27, 401–412.
Pegtel, D. M., & Gould, S. J. (2019).Exosomes. Annu Rev Biochem88,487–514.
Wang, Y., Liu, J., Ma, J., Sun, T., Zhou, Q., Wang, W., et al. (2019). Exosomal circRNAs: Biogenesis, effect and application in human diseases. Molecular Cancer, 18, 116.
Fanale, D., Taverna, S., Russo, A., & Bazan, V. (2018). Circular RNA in Exosomes. Advances In Experimental Medicine And Biology, 1087, 109–117.
Zhang, H., Deng, T., Ge, S., Liu, Y., Bai, M., Zhu, K., et al. (2019). Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene, 38, 2844–2859.
Shang, A., Gu, C., Wang, W., Wang, X., Sun, J., Zeng, B., et al. (2020). Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-beta1 axis. Molecular Cancer, 19, 117.
Wang, X., Zhang, H., Yang, H., Bai, M., Ning, T., Deng, T., et al. (2020). Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the mir-122-PKM2 axis in colorectal cancer. Molecular Oncology, 14, 539–555.
Shan, G., Shao, B., Liu, Q., Zeng, Y., Fu, C., Chen, A., et al. (2020). circFMN2 sponges miR-1238 to promote the expression of LIM-Homeobox gene 2 in prostate Cancer cells. Mol Ther Nucleic Acids, 21, 133–146.
Li, Y., Li, C., Xu, R., Wang, Y., Li, D., & Zhang, B. (2019). A novel circFMN2 promotes tumor proliferation in CRC by regulating the miR-1182/hTERT signaling pathways. Clin Sci (Lond), 133, 2463–2479.
Zhang, T., Yu, S., & Zhao, S. (2022). Hsa_circ_0005100 regulates tumorigenicity of colorectal carcinoma via miR-145-5p/MACC1 axis.J Clin Lab Anal36, e24533.
Li, L., Jiang, Z., Zou, X., & Hao, T. (2021). Exosomal circ_IFT80 enhances tumorigenesis and suppresses radiosensitivity in Colorectal Cancer by regulating miR-296-5p/MSI1 Axis. Cancer Manag Res, 13, 1929–1941.
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Research, 25, 981–984.
Bai, H., Lei, K., Huang, F., Jiang, Z., & Zhou, X. (2019). Exo-circRNAs: A new paradigm for anticancer therapy. Molecular Cancer, 18, 56.
Panda, A. C. (2018). Circular RNAs act as miRNA sponges. Advances In Experimental Medicine And Biology, 1087, 67–79.
Yang, G., Zhang, Y., & Yang, J. (2019). Identification of potentially functional CircRNA-miRNA-mRNA Regulatory Network in gastric carcinoma using Bioinformatics Analysis. Medical Science Monitor, 25, 8777–8796.
Liu, F., Fan, Y., Ou, L., Li, T., Fan, J., Duan, L., et al. (2020). CircHIPK3 facilitates the G2/M transition in prostate Cancer cells by sponging miR-338-3p. Onco Targets Ther, 13, 4545–4558.
Liu, H. S., Zheng, R. N., Guo, L. B., & Fu, X. J. (2020). Circular RNA circ_0000615 knockdown suppresses the development of nasopharyngeal cancer through regulating the miR-338-3p/FGF2 axis. Neoplasma, 67, 1032–1041.
Qian, W., Huang, T., & Feng, W. (2020). Circular RNA HIPK3 promotes EMT of Cervical Cancer through sponging mir-338-3p to Up-Regulate HIF-1alpha. Cancer Manag Res, 12, 177–187.
Zhang, W., Wang, B., Wang, Q., Zhang, Z., Shen, Z., Ye, Y., et al. (2020). Lnc-HSD17B11-1:1 functions as a competing endogenous RNA to promote Colorectal Cancer Progression by sponging mir-338-3p to Upregulate MACC1. Frontiers In Genetics, 11, 628.
Lu, M., Huang, H., Yang, J., Li, J., Zhao, G., Li, W., et al. (2019). Mir-338-3p regulates the proliferation, apoptosis and migration of SW480 cells by targeting MACC1. Exp Ther Med, 17, 2807–2814.
Zou, T., Duan, J., Liang, J., Shi, H., Zhen, T., Li, H., et al. (2018). Mir-338-3p suppresses colorectal cancer proliferation and progression by inhibiting MACC1. Int J Clin Exp Pathol, 11, 2256–2267.
Okano, H., Kawahara, H., Toriya, M., Nakao, K., Shibata, S., & Imai, T. (2005). Function of RNA-binding protein Musashi-1 in stem cells. Experimental Cell Research, 306, 349–356.
Chen, H., Liu, J., Wang, H., Cheng, Q., Zhou, C., Chen, X., et al. (2019). Inhibition of RNA-Binding protein Musashi-1 suppresses malignant Properties and Reverses Paclitaxel Resistance in Ovarian Carcinoma. Journal Of Cancer, 10, 1580–1592.
Lin, J. C., Tsai, J. T., Chao, T. Y., Ma, H. I., & Liu, W. H. (2019). Musashi-1 enhances Glioblastoma Migration by promoting ICAM1 translation. Neoplasia (New York, N.Y.), 21, 459–468.
Xiao, R., Yu, Y., Shen, S., Liu, F., & Kuang, R. (2019). Musashi1 promotes tumor metastasis and is a prognostic marker for renal carcinoma. Int J Clin Exp Pathol, 12, 313–319.
Liu, Q., Zhou, C., & Zhang, B. (2019). Upregulation of musashi1 increases malignancy of hepatocellular carcinoma via the Wnt/beta-catenin signaling pathway and predicts a poor prognosis. Bmc Gastroenterology, 19, 230.
Yang, L. Y., Song, G. L., Zhai, X. Q., Wang, L., Liu, Q. L., & Zhou, M. S. (2019). MicroRNA-331 inhibits development of gastric cancer through targeting musashi1. World Journal Of Gastrointestinal Oncology, 11, 705–716.
Chiou, G. Y., Yang, T. W., Huang, C. C., Tang, C. Y., Yen, J. Y., Tsai, M. C., et al. (2017). Musashi-1 promotes a cancer stem cell lineage and chemoresistance in colorectal cancer cells. Scientific Reports, 7, 2172.
Smith, A. R., Marquez, R. T., Tsao, W. C., Pathak, S., Roy, A., Ping, J., et al. (2015). Tumor suppressive microRNA-137 negatively regulates Musashi-1 and colorectal cancer progression. Oncotarget, 6, 12558–12573.
Acknowledgements
None.
Funding
This research was supported by the Natural Science Foundation of Hebei Province (H2020206311) and the Central Guiding Local Science and Technology Development Fund Project (Hebei Science and Technology Department Project) (226Z7712G).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The design of this protocol follows the tenets of the Declaration of Helsinki, approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University.
Animal experiments were approved by the Animal Ethics Committee of The Fourth Hospital of Hebei Medical University.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
#Co-first author.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
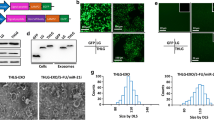
The Detection of the Stability and size of Exosomes. (A-B) NTA Analysis was used to Assess the size of Exo-HV and Exo-CRC. (C) The expression of exosomal circ_FMN2 was examined by qRT-PCR after placing blood samples at room temperature 0 h, 6 h, 12 h, and 24 h. (D) The expression of exosomal circ_FMN2 was determined by qRT-PCR after freezing and thawing blood samples repeatedly 0 cycle, 2 cycles, 4 cycles and 8 cycles.
Supplementary Fig. 2
The representative pictures for Fig. 2I (A), 2 J-K (B-C), 4I-J (D-E) and 6I-J (F-G).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Q., Zhang, Y., Tian, Y. et al. Exosomal Circ_FMN2 Derived from the Serum of Colorectal Cancer Patients Promotes Cancer Progression by miR-338-3p/MSI1 Axis. Appl Biochem Biotechnol 195, 7322–7337 (2023). https://doi.org/10.1007/s12010-023-04456-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04456-3