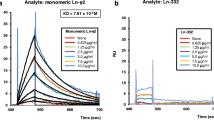
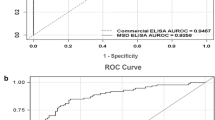
Abstract
Interleukin-18 (IL-18), a member of IL-1 cytokine superfamily, is deemed as an important indicator of the kidney disease. Herein a sandwich chemiluminescence immunoassay integrated with magnetic beads was conducted to detect IL-18 in kidney disease. The detection limit and linear range were 0.0044 ng/mL and 0.01–2.7 ng/mL, respectively. Satisfactory recoveries were ranged from 91.70 to 101.18% with the relative standard deviation below 10%; interference bias of most biomarkers were within allowable deviation range (± 15%). In summary, the whole study was successfully applied to detect IL-18 levels in urine samples for patients with kidney disease. The results showed that chemiluminescence immunoassay for IL-18 detection could be used in the clinical application.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- CLIA:
-
Chemiluminescence method
- CKD:
-
Chronic kidney disease
- AKI:
-
Acute kidney injury
- sCr:
-
Serum creatinine
- KIM-1:
-
Kidney injury molecule 1
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- L-FABP:
-
Liver-type fatty acid-binding protein
- NAG:
-
N-acetyl-β-d-glucosaminidase
- TIMP-2:
-
Tissue inhibitor of metallopmteinase-2
- IL-18:
-
Interleukin-18
- BMG:
-
β2-Microglobulin
- CysC:
-
Cystatin C
- RBP:
-
Retinol-binding protein
- ALB:
-
Albumin
- AMG:
-
α1-Microglobulin
- TRF:
-
Transferrin
- BSA:
-
Bovine serum albumin
- EDC:
-
1-Ethyl-3-(3-dimethylaminopropyl)-carbodimide hydrochloride
- NHS:
-
N-Hydroxysuccinimide
- DMSO:
-
Dimethyl sulfoxide
- DMF:
-
N,N-dimethylformamide
- MES:
-
4-Morpholineethanesulfonicacid
- SMCC:
-
4-(N-Maleimidomethyl) cyclohexanecarboxylic acid N-hydroxysuccinimide ester
- SD:
-
Standard deviation,
- CV:
-
Coefficient variation
References
Nolan, N., Valdivieso, K., Mani, R., Yang, J. Y. C., Sarwal, R. D., & Katzenbach, P. (2020). Clinical and analytical validation of a novel urine-based test for the detection of allograft rejection in renal transplant patients. Journal of Clinical Medicine, 9, 2325.
Bullich, G., Domingo-Gallego, A., Vargas, I., Ruiz, P., Lorente-Grandoso, L., & Furlano, M. (2018). A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney International, 94, 363–371.
Berni, E., Pritchard, N., Jenkins-Jones, S., Ambery, P., Jain, M., & Jermutus, L. (2018). Hospital admissions for severe infections in people with chronic kidney disease in relation to renal disease severity and diabetes status. Endocrinology, Diabetes and Metabolism, 1, e00029.
Panahi, M. H., Hadaegh, F., Yavari, P., Kazempour-Ardebili, S., Mehrabi, Y., & Azizi, F. (2016). A challenging interaction of chronic kidney disease with other metabolic disorders. Iranian Journal of Kidney Diseases, 10, 274–281.
Zeng, X., Hossain, D., Bostwick, D. G., Herrera, G. A., & Zhang, P. L. (2014). Urinary β2-microglobulin is a good indicator of proximal tubule injury: A correlative study with renal biopsies. Journal of Biomarkers, 2014, 1–7.
Abulaban, K. M., Song, H., Zhang, X., Kimmel, P. L., Kusek, J. W., & Nelson, R. G. (2016). Predicting decline of kidney function in lupus nephritis using urine biomarkers. Lupus, 25, 1012–1018.
Herget, R. S., Marggraf, G., & Hüsing, J. (2004). Early detection of acute renal failure by serum cystatin C. Kidney International, 66(3), 1115–1122.
Khreba, N. A., Abdelsalam, M., Wahab, A. M., Sanad, M., Elhelaly, R., & Adel, M. (2019). Kidney injury molecule 1 (KIM-1) as an early predictor for acute kidney injury in post-cardiopulmonary bypass (CPB) in open heart surgery patients. International Journal of Nephrology, 2019, 1–6.
Allegretti, A. S., Parada, X. V., Endres, P., Zhao, S., Krinsky, S., St. Hillien, & S. A., (2021). Urinary NGAL as a diagnostic and prognostic marker for acute kidney injury in cirrhosis: A prospective study. Clinical and Translational Gastroenterology, 12, e00359.
Torigoe, K., Muta, K., Tsuji, K., Yamashita, A., Ota, Y., & Kitamura, M. (2020). Urinary liver-type fatty acid-binding protein predicts residual renal function decline in patients on peritoneal dialysis. Medical Science Monitor, 26, e928236.
Ramesh, G., Kwon, O., & Ahn, K. (2010). Netrin-1: A novel universal biomarker of human kidney injury. Transplantation Proceedings, 42, 1519–1522.
Wohlfahrtova, M., Brabcova, I., Zelezny, F., Balaz, P., Janousek, L., & Honsova, E. (2014). Tubular atrophy and low netrin-1 gene expression are associated with delayed kidney allograft function. Transplantation, 97, 176–183.
Kaufmann, M., Schlossbauer, M., Hubauer, U., Stadler, S., Fischer, M., & Wallner, S. (2020). N-acety-b-D-glucosaminidase: A potential biomarker for early detection of acute kidney injury in acute chest pain. Nephrology, 25, 135–143.
Gunnerson, K. J., Shaw, A. D., Chawla, L. S., Bihorac, A., Al-Khafaji, A., & Kashani, K. (2016). TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients.. Journal of Trauma and Acute Care, 80, 243–249.
Nariai, Y., Kamino, H., Obayashi, E., Kato, H., Sakashita, G., & Sugiura, T. (2019). Generation and characterization of antagonistic anti-human interleukin (IL)-18 monoclonal antibodies with high affinity: Two types of monoclonal antibodies against full-length IL-18 and the neoepitope of inflammatory caspase-cleaved active IL-18. Arch. Archives of Biochemistry and Biophysics, 663, 71–82.
Sueud, T., Hadi, N. R., Abdulameer, R., Jamil, D. A., & Al-Aubaidy, H. A. (2019). Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. Diabetes & Metabolic Syndrome, 13, 564–568.
Xiao, M., Bolduc, D. L., Li, X., Cui, W., Hieber, K. P., & Bünger, R. (2017). Urine interleukin-18 (IL-18) as a biomarker of total-body irradiation: A preliminary study in nonhuman primates. Radiation Research, 188, 325.
Yasin, S., Fall, N., Brown, R. A., Henderlight, M., & Canna, S. W. (2020). IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology, 59, 361–366.
Altendahl, M., Maillard, P., Harvey, D., Cotter, D., Walters, S., & Wolf, A. (2020). An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury. PLoS ONE, 15, e0227835.
Tsai, M. H., Chen, Y. C., Yang, C. W., Jenq, C. C., Fang, J. T., & Lien, J. M. (2013). Acute renal failure in cirrhotic patients with severe sepsis: value of urinary interleukin-18. Journal of Gastroenterology and Hepatology, 28, 135–141.
Wang, C. G., Zhang, J. H., Han, J. L., Yang, Q. Y., Liu, J. R., & Liang, B. (2017). The level of urinary IL-18 in acute kidney injury after cardiopulmonary bypass. Experimental and Therapeutic Medicine, 14(6), 6047–6051.
Lin, X., Yuan, J., Zhao, Y. T., & Zha, Y. (2015). Urine interleukin-18 in prediction of acute kidney injury: A systemic review and meta-analysis. Journal of Nephrology, 28, 7–16.
Hall, I. E., Yarlagadda, S. G., Coca, S. G., Wang, Z., Parikh, C. R. (2010). IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. Journal of the American Society of Nephrology, 21(1), 189–197.
Rasoulzadeh, F., Amjadi, M., & Ghorbani, M. (2020). A highly sensitive chemiluminescence assay for diniconazole by using CuInS2 quantum dots. Microchemical Journal, 159, 105323.
Kim, J., Kim, J., Rho, T., & Lee, J. H. (2014). Rapid chemiluminescent sandwich enzyme immunoassay capable of consecutively quantifying multiple tumor markers in a sample. Talanta, 129, 106–112.
Harada, M., Nagai, J., Kurata, R., Shimizu, K., Cui, X., & Isagawa, T. (2020). Establishment of novel high-standard chemiluminescent assay for NTPase in two protozoans and its high-throughput screening. Marine Drugs, 18, 161.
Sun, Y., Dai, Y., Zhu, X., Han, R., Wang, X., & Luo, C. (2020). A nanocomposite prepared from bifunctionalized ionic liquid chitosan, graphene oxide and magnetic nanoparticles for aptamer-based assay of tetracycline by chemiluminescence. Microchimica Acta, 187, 63.
He, S., Yang, M., Wu, X., Cai, G., Jiang, K., & Xie, L. (2020). Comparison of a novel chemiluminescence immunoassay with the passive agglutination method for the diagnosis of Mycoplasma pneumoniae infection in children. Journal of Microbiological Methods, 173, 105921.
Sathishkumar, N., & Toley, B. J. (2020). Development of an experimental method to overcome the hook effect in sandwich-type lateral flow immunoassays guided by computational modelling. Sensors and Actuators B: Chemical, 324, 128756.
Lok, C., Frijstein, M., & Van Trommel, N. (2021). Clinical presentation and diagnosis of gestational trophoblastic disease. Best Practice & Research Clinical Obstetrics & Gynaecology, 74, 42–52.
Mei, Y., Li, C., Yang, S., Chen, W., Liu, R., & Xu, K. (2021). A novel biosensor for the rapid and high-sensitive detection of mercury based on cleavable phosphorothioate RNA probe. Sensors and Actuators B: Chemical, 337, 129756.
Funding
This work was supported by grants from National Natural Science Foundation of China (81860399), the Provincial Youth Science Fund of Jiangxi Province (20142BAB215072), and the Science and Technology Key R&D Program of Jiangxi Province (20202BBG72001).
Author information
Authors and Affiliations
Contributions
Xiaoling Fu and Lanya Li designed the study and drafted the manuscript; Guang Wu, Kaike Tang, and Jian Zhang assisted in patient recruitment, provided clinical treatments, and contributed critical views the study. Zhitian Chen and Mingjin Shi performed the experiments and data analysis, and supervised the study. Bo Zhang critically reviewed the manuscript. All co-authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of the Shuyang Hospital of Traditional Chinese Medicine (Ethics number: 20210202–001) and written informed consent was obtained from all the patients.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, X., Li, L., Wu, G. et al. Establishment of Sensitive Sandwich-Type Chemiluminescence Immunoassay for Interleukin-18 in Urinary Samples. Appl Biochem Biotechnol 195, 7414–7428 (2023). https://doi.org/10.1007/s12010-023-04453-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04453-6