Abstract
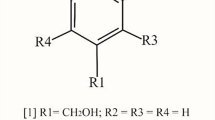
Diabetes-associated postprandial hyperglycemia is a major risk factor in cardiovascular disease. Since enzyme α-glucosidase is primarily responsible for glucose release during digestion, inhibiting it mitigates post-meal spike in blood glucose level. Metabolites from endophytic fungi could be potential natural inhibitors of this enzyme. Endophytic fungi isolated from Bauhinia purpurea L. were screened for their potential antioxidant and antidiabetic activities. Ethyl acetate extract of Nigrospora sphaerica BRN 01 (NEE) displayed high antioxidant activity with an IC50 value of 9.72 ± 0.91 µg/ml for DPPH assay and ferric reducing antioxidant power (FRAP) of 1595 ± 0.23 µmol AAE g−1 DW. NEE also showed high degree of inhibition of α-glucosidase activity with an IC50 value of 0.020 ± 0.001 mg/ml, significantly greater than the standard drug acarbose (0.494 ± 0.009 mg/ml). Metabolite profiling of NEE was carried using ultra-high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry (UHPLC-ESI-QTOF-MS) and 21 metabolites identified based on the MS/MS fragmentation patterns. Docking analysis of all 21 identified metabolites was carried out. Of these, 6 showed binding energies higher than acarbose (− 6.6 kcal/mol). Based on the analysis of interactions of feruloyl glucose with active site residues of the enzyme, it could be a potential α-glucosidase inhibitor. Metabolites of Nigrospora sphaerica BRN 01, therefore, could be potential lead molecules for design and development of antidiabetic drugs.






Similar content being viewed by others
Data Availability
Data will be made available on request.
References
IDF Diabetes Atlas | Tenth Edition. (n.d.). Retrieved from https://diabetesatlas.org/
Hossain, U., Das, A. K., Ghosh, S., & Sil, P. C. (2020). An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food and Chemical Toxicology, 145, 111738. https://doi.org/10.1016/J.FCT.2020.111738
Riyaphan, J., Pham, D. C., Leong, M. K., & Weng, C. F. (2021). In silico approaches to identify polyphenol compounds as α-glucosidase and α-amylase inhibitors against type-II diabetes. Biomolecules, 11(12), 1877. https://doi.org/10.3390/BIOM11121877
Akmal, M., & Wadhwa, R. (2022). Alpha glucosidase inhibitors. NCBI Bookshelf. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK557848/
Smith, D. L., Orlandella, R. M., Allison, D. B., & Norian, L. A. (2021). Diabetes medications as potential calorie restriction mimetics—A focus on the alpha-glucosidase inhibitor acarbose. GeroScience, 43(3), 1123–1133. https://doi.org/10.1007/S11357-020-00278-X/FIGURES/1
Uuh Narvaez, J. J., & Segura Campos, M. R. (2022). Combination therapy of bioactive compounds with acarbose: A proposal to control hyperglycemia in type 2 diabetes. Journal of Food Biochemistry, e14268. https://doi.org/10.1111/JFBC.14268
Dirir, A. M., Daou, M., Yousef, A. F., & Yousef, L. F. (2021). A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochemistry Reviews, 21(4), 1049–1079. https://doi.org/10.1007/S11101-021-09773-1
Wen, J., Okyere, S. K., Wang, S., Wang, J., Xie, L., Ran, Y., & Hu, Y. (2022). Endophytic fungi: An effective alternative source of plant-derived bioactive compounds for pharmacological studies. Journal of Fungi, 8(2), 205. https://doi.org/10.3390/JOF8020205
Benjamim, J., da Costa, K., Santos, A., & Santos, A. (2021). Chemical, botanical and pharmacological aspects of the Leguminosae. Pharmacognosy Reviews, 14(28), 106–120. https://doi.org/10.5530/phrev.2020.14.15
Xu, T., Song, Z., Hou, Y., Liu, S., Li, X., Yang, Q., & Wu, S. (2022). Secondary metabolites of the genus Nigrospora from terrestrial and marine habitats: Chemical diversity and biological activity. Fitoterapia, 161, 105254. https://doi.org/10.1016/J.FITOTE.2022.105254
Suryanarayanan, T. S., Kumaresan, V., & Johnson, J. A. (1998). Foliar fungal endophytes from two species of the mangrove Rhizophora. Canadian Journal of Microbiologyicrobiology, 44(10), 1003–1006. https://doi.org/10.1139/W98-087
Sharma, D., Pramanik, A., & Agrawal, P. K. (2016). Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3 Biotech, 6(2). https://doi.org/10.1007/S13205-016-0518-3
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular biology and evolution, 35(6), 1547–1549. https://doi.org/10.1093/MOLBEV/MSY096
Tamura, K., Nei, M., & Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences of the United States of America, 101(30), 11030–11035. https://doi.org/10.1073/PNAS.0404206101/ASSET/D0B973E3-6167-4566-B866-7A0FB0A0057B/ASSETS/GRAPHIC/ZPQ0300455080006.JPEG
Bulut, O., Akın, D., Sönmez, Ç., Öktem, A., Yücel, M., & Öktem, H. A. (2019). Phenolic compounds, carotenoids, and antioxidant capacities of a thermo-tolerant Scenedesmus sp. (Chlorophyta) extracted with different solvents. Journal of Applied Phycology, 31(3), 1675–1683. https://doi.org/10.1007/S10811-018-1726-5/TABLES/4
Khor, B.-K., Jeng-YeouChear, N., Azizi, J., Khaw, K.-Y., Khor, B.-K., Chear, N., & Chemical, K.-Y. (2021). Chemical composition, antioxidant and cytoprotective potentials of Carica papaya leaf extracts: A comparison of supercritical fluid and conventional extraction methods. Molecules, 26(5), 1489. https://doi.org/10.3390/MOLECULES26051489
Bhatia, A., Singh, B., Arora, R., & Arora, S. (2019). In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complementary and Alternative Medicine, 19(1). https://doi.org/10.1186/s12906-019-2482-z
Biswal, R. P., Dandamudi, R. B., Patnana, D. P., Pandey, M., & Vutukuri, V. N. R. K. (2022). Metabolic fingerprinting of Ganoderma spp using UHPLC-ESI-QTOF-MS and its chemometric analysis. Phytochemistry, 199, 113169. https://doi.org/10.1016/J.PHYTOCHEM.2022.113169
Sim, L., Quezada-Calvillo, R., Sterchi, E. E., Nichols, B. L., & Rose, D. R. (2008). Human intestinal maltase–glucoamylase: Crystal structure of the N-terminal catalytic subunit and basis of inhibition and substrate specificity. Journal of Molecular Biology, 375(3), 782–792. https://doi.org/10.1016/J.JMB.2007.10.069
Seeliger, D., & De Groot, B. L. (2010). Conformational transitions upon ligand binding: Holo-structure prediction from apo conformations. PLoS Computational Biology, 6(1). https://doi.org/10.1371/JOURNAL.PCBI.1000634
Trott, O., & Olson, A. J. (2010). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455–461. https://doi.org/10.1002/JCC.21334
Ghanta, P., Sinha, S., Doble, M., & Ramaiah, B. (2020). Potential of pyrroquinazoline alkaloids from Adhatoda vasica Nees. as inhibitors of 5-LOX – a computational and an in-vitro study. https://doi.org/10.1080/07391102.2020.1848635, 40(6), 2785–2796. https://doi.org/10.1080/07391102.2020.1848635
Hyun, T. K., Kim, H. C., & Kim, J. S. (2014). Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Industrial Crops & Products, Complete(52), 611–616. https://doi.org/10.1016/J.INDCROP.2013.11.039
Giacco, F., & Brownlee, M. (2010). Oxidative stress and diabetic complications. Circulation research, 107(9), 1058. https://doi.org/10.1161/CIRCRESAHA.110.223545
Erenler, R., Telci, I., Ulutas, M., Demirtas, I., Gul, F., Elmastas, M., & Kayir, O. (2015). Chemical constituents, quantitative analysis and antioxidant activities of Echinacea purpurea (L.) Moench and Echinacea pallida (Nutt.) Nutt. Journal of Food Biochemistry, 39(5), 622–630. https://doi.org/10.1111/JFBC.12168
Erenler, R., Sen, O., Aksit, H., Demirtas, I., Yaglioglu, A. S., Elmastas, M., & Telci, I. (2016). Isolation and identification of chemical constituents from Origanum majorana and investigation of antiproliferative and antioxidant activities. Journal of the Science of Food and Agriculture, 96(3), 822–836. https://doi.org/10.1002/JSFA.7155
Visavadiya, N. P., Soni, B., & Dalwadi, N. (2009). Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. International Journal of Food Sciences and Nutrition, 60(SUPPL. 2), 135–149. https://doi.org/10.1080/09637480902877998
Rigane, G., Ghazghazi, H., Aouadhi, C., Ben Salem, R., & Nasr, Z. (2017). Phenolic content, antioxidant capacity and antimicrobial activity of leaf extracts from Pistacia atlantica. Natural Product Research, 31(6), 696–699. https://doi.org/10.1080/14786419.2016.1212035
Nadeem, M., Mumtaz, M. W., Danish, M., Rashid, U., Mukhtar, H., & Irfan, A. (2020). Antidiabetic functionality of Vitex negundo L. leaves based on UHPLC-QTOF-MS/MS based bioactives profiling and molecular docking insights. Industrial Crops and Products, 152, 112445. https://doi.org/10.1016/J.INDCROP.2020.112445
Supaphon, P., & Preedanon, S. (2019). Evaluation of in vitro alpha-glucosidase inhibitory, antimicrobial, and cytotoxic activities of secondary metabolites from the endophytic fungus, Nigrospora sphaerica, isolated from Helianthus annuus. Annals of Microbiology, 69(13), 1397–1406. https://doi.org/10.1007/S13213-019-01523-1/TABLES/3
Sharaf, M. H., Abdelaziz, A. M., Kalaba, M. H., Radwan, A. A., & Hashem, A. H. (2022). Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from Ocimum Basilicum. Applied Biochemistry and Biotechnology, 194(3), 1271–1289. https://doi.org/10.1007/S12010-021-03702-W/FIGURES/6
Robert, J. -, & Yardley, V. (2009). Drugs for neglected diseases initiative (DNDi). Swiss Tropical Institute (STI), Tanja Wenzler, Swiss Tropical Institute (STI): Reto Brun.
Ruttkies, C., Schymanski, E. L., Wolf, S., Hollender, J., & Neumann, S. (2016). MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. Journal of Cheminformatics, 8(1). https://doi.org/10.1186/S13321-016-0115-9
Nadeem, M., Mumtaz, M. W., Danish, M., Rashid, U., Mukhtar, H., & Irfan, A. (2020). Antidiabetic functionality of Vitex negundo L. leaves based on UHPLC-QTOF-MS/MS based bioactives profiling and molecular docking insights. Industrial Crops and Products, 152(April), 112445. https://doi.org/10.1016/j.indcrop.2020.112445
Tanaka, T., Tanaka, T., & Tanaka, M. (2011). Potential cancer chemopreventive activity of protocatechuic acid. Journal of Experimental and Clinical Medicine, 3(1), 27–33. https://doi.org/10.1016/J.JECM.2010.12.005
Adefegha, S. A., Oboh, G., Ejakpovi, I. I., & Oyeleye, S. I. (2015). Antioxidant and antidiabetic effects of gallic and protocatechuic acids: A structure–function perspective. Comparative Clinical Pathology, 24(6), 1579–1585. https://doi.org/10.1007/S00580-015-2119-7/FIGURES/7
Kramberger, K., Barlič-Maganja, D., Bandelj, D., BarucaArbeiter, A., Peeters, K., MiklavčičVišnjevec, A., & Pražnikar, Z. J. (2020). HPLC-DAD-ESI-QTOF-MS determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species. Metabolites, 10(10), 1–25. https://doi.org/10.3390/METABO10100403
Chen, S., Liu, Z., Li, H., Xia, G., Lu, Y., He, L., & She, Z. (2015). β-Resorcylic acid derivatives with α-glucosidase inhibitory activity from Lasiodiplodia sp. ZJ-HQ1, an endophytic fungus in the medicinal plant Acanthus ilicifolius. Phytochemistry Letters, 13, 141–146. https://doi.org/10.1016/j.phytol.2015.05.019
Huang, D. Y., Nong, X. H., Zhang, Y. Q., Xu, W., Sun, L. Y., Zhang, T., … Han, C. R. (2021). Two new 2,5-diketopiperazine derivatives from mangrove-derived endophytic fungus Nigrospora camelliae-sinensis S30. https://doi.org/10.1080/14786419.2021.1878168. https://doi.org/10.1080/14786419.2021.1878168
Zhang, F., Li, B., Wen, Y., Liu, Y., Liu, R., Liu, J., & Jiang, Y. (2022). An integrated strategy for the comprehensive profiling of the chemical constituents of Aspongopus chinensis using UPLC-QTOF-MS combined with molecular networking. Pharmaceutical Biology, 60(1), 1349. https://doi.org/10.1080/13880209.2022.2096078
Lan, H. C., Li, S. Z., Li, K., & Liu, E. H. (2021). In vitro human intestinal microbiota biotransformation of nobiletin using liquid chromatography–mass spectrometry analysis and background subtraction strategy. Journal of Separation Science, 44(10), 2046–2053. https://doi.org/10.1002/JSSC.202001150
Zhang, H., Deng, Z., Guo, Z., Tu, X., Wang, J., & Zou, K. (2014). Pestalafuranones F-J, Five new furanone analogues from the endophytic fungus Nigrospora sp. BM-2. Molecules, 19(1), 819–825. https://doi.org/10.3390/MOLECULES19010819
Shen, Y., Liu, X., Yang, Y., Li, J., Ma, N., & Li, B. (2015). In vivo and in vitro metabolism of aspirin eugenol ester in dog by liquid chromatography tandem mass spectrometry. Biomedical Chromatography, 29(1), 129–137. https://doi.org/10.1002/BMC.3249
Zhang, X., Jia, Y., Ma, Y., Cheng, G., & Cai, S. (2018). Phenolic Composition, antioxidant properties, and inhibition toward digestive enzymes with molecular docking analysis of different fractions from Prinsepia utilis Royle fruits. Molecules, 23(12), 3373. https://doi.org/10.3390/MOLECULES23123373
Acknowledgements
The authors are ever grateful to the Founder Chancellor, Sri Sathya Sai Institute of Higher Learning (SSSIHL), Bhagawan Sri Sathya Sai Baba, for constant guidance and support. The authors are thankful to SSSIHL and UGC-SAP for providing financial support to carry out this work. The authors are grateful to Central Research Instrumentation Facility (CRIF), SSSIHL, for providing all necessary facilities. The authors wish to thank V. N. Ravi Kishore Vutukuri for the help provided in optimizing mass spectrometry protocols.
Author information
Authors and Affiliations
Contributions
Sai Anand Kannakazhi Kantari—development of methodologies and conducting investigations; data analysis and data presentation and writing the original draft.
Ranendra Pratap Biswal—development of methodology for LC–MS analysis.
Piyush Kumar—participated in conducting investigations.
Malleswara Dharanikota—providing some resources.
Ashok Agraharam—conception or design of the work, writing (review and editing), supervision.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kantari, S.A.K., Biswal, R.P., Kumar, P. et al. Antioxidant and Antidiabetic Activities, and UHPLC-ESI-QTOF-MS-Based Metabolite Profiling of an Endophytic Fungus Nigrospora sphaerica BRN 01 Isolated from Bauhinia purpurea L. Appl Biochem Biotechnol 195, 7465–7482 (2023). https://doi.org/10.1007/s12010-023-04452-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04452-7