Abstract
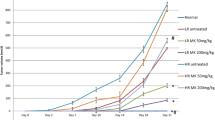
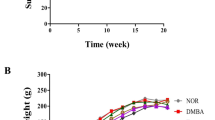
Exploration of new strategies and identification of less expensive novel chemoprevention agents against breast cancer progression have become the need of the hour. Thus, the present study aimed at evaluating the anti-cancer efficacies of octyl gallate (OG) and gallic acid (GA) isolated from Terminalia bellirica (T. bellirica) in breast cancer cell lines and DMBA-induced Sprague–Dawley animal model. The results of western blot analysis show significant (p < 0.05) downregulation of anti-apoptotic protein (Bcl-2 and Bcl-xL) expression and up-regulation of pro-apoptotic protein (Bak and Bax) expression in both MCF-7 and MDA-MB-231 cell lines. Our findings also show that DMBA-induced Sprague–Dawley rats (50–55 days old) orally administered with OG (20 mg/kg body wt.) and GA (20 mg/kg body wt.) for a treatment period of 14 weeks were observed for normalized body weight changes and hematological indices and significant reduction of tumor markers carcinoembryonic antigen (CEA), cancer antigen 15.3 (CA 15.3), and oxidative stress (TBARS) in serum, while the activity of anti-oxidant enzyme (SOD, CAT, and GPx) levels estimated in the mammary tissue was found restored back to normal. Computational molecular interaction study was also performed to substantiate the in vitro obtained results. The tissue histology reveals the therapeutic role of OG and GA. The study conducted brings to limelight of the molecular mechanisms of intrinsic apoptotic signaling pathway through which OG and GA exert their chemopreventive action. Both OG and GA can be explored further as chemotherapeutic natural drugs for their ability to prevent breast cancer progression.









Similar content being viewed by others
References
Dolatkhah, R., Somi, M. H., Jafarabadi, M. A., Hosseinalifam, M., Sepahi, S., Belalzadeh Nezamdoust, M., and Dastgiri, S. (2020). Breast cancer survival and incidence: 10 years cancer registry data in the Northwest. International Journal of Breast Cancer, 1, 1963814. https://doi.org/10.1155/2020/1963814
Fitzmaurice, C. (2018). The Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncology, 4(11), 1553–1568.
Pfeffer, C. M., and Singh, A. T. K. (2018). Apoptosis: A target for anticancer therapy. International Journal of Molecular Sciences, 19(2), 448. https://doi.org/10.3390/ijms19020448
Haldar, S., Chintapalli, J., and Croce, C. M. (1996). Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Research, 56(6), 1253–1255.
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D. M., Forman, D., and Bray, F. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359–E386. https://doi.org/10.1002/ijc.29210
Padmini, R., Uma Maheshwari, V., Saravanan, P., Woo Lee, K., Razia, M., Alwahibi, M. S., Ravindran, B., Soliman Elshikh, M., Ock Kim, Y., Kim, H., and Kim, H. J. (2020). Identification of novel bioactive molecules from garlic bulbs: A special effort to determine the anticancer potential against lung cancer with targeted drugs. Saudi Journal of Biological Science, 27(12), 3274–3289. https://doi.org/10.1016/j.sjbs.2020.09.041
Weigelt, B., Horlings, H. M., Kreike, B., Hayes, M. M., Hauptmann, M., Wessels, L. F., de Jong, D., Van de Vijver, M. J., Van’t Veer, L. J., and Peterse, J. L. (2008). Refinement of breast cancer classification by molecular characterization of histological special types. Journal of Pathology, 216(2), 141–150. https://doi.org/10.1002/path.2407
Zaman, S., Wang, R., and Gandhi, V. (2014). Targeting the apoptosis pathway in hematologic malignancies. Leukaemia and Lymphoma, 55(9), 1980–1992. https://doi.org/10.3109/10428194.2013.855307
Strasser, A., Cory, S., and Adams, J. M. (2011). Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO Journal, 30(18), 3667–3683. https://doi.org/10.1038/emboj.2011.307
Yin, S. Y., Wei, W. C., Jian, F. Y., and Yang, N. S. (2013). Therapeutic applications of herbal medicines for cancer patients. Evidence-Based Complementary and Alternative Medicine, 2013, 302426. https://doi.org/10.1155/2013/302426
Huang, Y. T., Wen, C. C., Chen, Y. H., Huang, W. C., Huang, L. T., Lin, W. C., Arulselvan, P., Liao, J. W., Lin, S. H., Hsiao, P. W., Kuo, S. C., and Yang, N. S. (2013). Dietary uptake of Wedelia chinensis extract attenuates dextran sulfate sodium-induced colitis in mice. PLoS One, 8(5), e64152. https://doi.org/10.1371/journal.pone.0064152
Lee, J. K., Kim, J. H., and Shin, H. K. (2011). Therapeutic effects of the oriental herbal medicine Sho-saiko-to on liver cirrhosis and carcinoma. Hepatology Research, 41(9), 825–837. https://doi.org/10.1111/j.1872-034X.2011.00829.x
Lam, W., Bussom, S., Guan, F., Jiang, Z., Zhang, W., Gullen, E. A., Liu, S. H., and Cheng, Y. C. (2010). The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Science Translational Medicine, 2(45), 45ra59. https://doi.org/10.1126/scitranslmed.3001270
Sales, M. S., Roy, A., Antony, L., Banu, S. K., Jeyaraman, S., and Manikkam, R. (2018). Octyl gallate and gallic acid isolated from Terminalia bellarica regulates normal cell cycle in human breast cancer cell lines. Biomedicine and Pharmacotherapy, 103, 1577–1584. https://doi.org/10.1016/j.biopha.2018.04.182
Qi, F., Li, A., Inagaki, Y., Gao, J., Li, J., Kokudo, N., Li, X. K., and Tang, W. (2010). Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. BioScience Trends, 4(6), 297–307.
Latha, R. C., and Daisy, P. (2011). Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chemico-Biological Interactions, 189(1–2), 112–118. https://doi.org/10.1016/j.cbi.2010.11.005
Patra, S., Panda, P. K., Naik, P. P., Panigrahi, D. P., Praharaj, P. P., Bhol, C. S., Mahapatra, K. K., Padhi, P., Jena, M., Patil, S., Patra, S. K., and Bhutia, S. K. (2020). Terminalia bellirica extract induces anticancer activity through modulation of apoptosis and autophagy in oral squamous cell carcinoma. Food and Chemical Toxicology, 136, 111073. https://doi.org/10.1016/j.fct.2019.111073
Basu, T., Panja, S., Ghate, N. B., Chaudhuri, D., and Mandal, N. (2017). Antioxidant and antiproliferative effects of different solvent fractions from Terminalia belerica Roxb. fruit on various cancer cells. Cytotechnology, 69(2), 201–216.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193(1), 265–275.
Khan, R. S., Senthi, M., Rao, P. C., Basha, A., Alvala, M., Tummuri, D., Masubuti, H., Fujimoto, Y., and Begum, A. S. (2015). Cytotoxic constituents of Abutilon indicum leaves against U87MG human glioblastoma cells. Natural Product Research, 29(11), 1069–1073. https://doi.org/10.1080/14786419.2014.976643
Musthafa, S. A., Kasinathan, T., Bhattacharyya, R., Muthu, K., Kumar, S., and Munuswamy-Ramanujam, G. (2021). Gallic acid synergistically enhances the apoptotic ability of abutilon indicum Linn. Stem fraction inhuman U87 glioblastoma cells. Materials Today: Proceedings, 40, S216–S223. https://doi.org/10.1016/j.matpr.2020.10.285
Verma, D., Tiwari, S., and Rawat, A. (2011). Pharmacognostical Evaluation and Phytochemical Standardization of Abrus precatorius L. seeds. Natural Product Sciences, 17, 51–57.
Ramli, S., Harada, K. I., and Ruangrungsi, N. (2011). Antioxidant, antimicrobial and cytotoxicity activities of Acacia farnesiana (L.) Willd. leaves ethanolic extract. Pharmacognosy Journal, 3(23), 50–58.
Claudia, D. P., Mario, C. H., Arturo, N. O., Omar Noel, M. C., Antonio, N. C., Teresa, R. A., Zenón Gerardo, L. T., Margarita, D. M., Marsela Alejandra, Á. I., Yessica Rosalina, C. M., Vanessa, S. Q., Francisco Enrique, G., Iván, T. V., Janette, F. C., María Del Rayo, C. C., and José, P. C. (2018). Phenolic compounds in organic and aqueous extracts from Acacia farnesiana Pods analyzed by ULPS-ESI-Q-oa/TOF-MS. In vitro antioxidant activity and anti-inflammatory response in CD-1 mice. Molecules, 23(9), 2386. https://doi.org/10.3390/molecules23092386
Foyzun, T., Mahmud, A. A., Ahammed, M. S., Manik, M. I. N., Hasan, M. K., Islam, K. M. M., Lopa, S. S., Al-Amin, M. Y., Biswas, K., Afrin, M. R., Alam, A. K., and Sadik, G. (2022). Polyphenolics with strong antioxidant activity from Acacia nilotica ameliorate some biochemical signs of arsenic-induced neurotoxicity and oxidative stress in mice. Molecules, 27(3), 1037. https://doi.org/10.3390/molecules27031037
Andonova, T., Muhovski, Y., Slavov, I., Vrancheva, R., Georgiev, V., Apostolova, E., Naimov, S., Mladenov, R., Pavlov, A., and Dimitrova-Dyulgerova, I. (2023). Phenolic profile, antioxidant and DNA-protective capacity, and microscopic characters of Ailanthus altissima aerial substances. Plants, 12(4), 920.
Baptista, A. B., Sarandy, M. M., Gonçalves, R. V., Novaes, R. D., Gonçalves da Costa, C., Leite, J. P. V., and Peluzio, M. D. C. G. (2020). Antioxidant and anti-inflammatory effects of Anacardium occidentale L. and Anacardium microcarpum D. extracts on the liver of IL-10 knockout mice. Evid Based Complement Alternative Medicine, 2020, 3054521. https://doi.org/10.1155/2020/3054521
Encarnação, S., De Mello-Sampayo, C., Carrapiço, B., São Braz, B., Jordão, A. P., Peleteiro, C., Catarino, L., Silva, I. B. M. D., Gouveia, L. F., Lima, B. S., and Silva, O. (2022). Plants (Basel), 11(19), 2637. https://doi.org/10.3390/plants11192637
Bagul, M. S., Ravishankara, M. N., Padh, H., and Rajani, M. (2003). Phytochemical evaluation and free radical scavenging properties of rhizome of Bergenia ciliata (Haw.) Sternb. Forma ligulata Yeo. Journal of Natural Remedies, 3(1), 83–89. https://doi.org/10.18311/jnr/2003/369
Kumar, T., and Jain, V. (2014). Antinociceptive and anti-inflammatory activities of Bridelia retusa methanolic fruit extract in experimental animals. Scientific World Journal, 2014, 890151. https://doi.org/10.1155/2014/890151
Insanu, M., Karimah, H., Pramastya, H., and Fidrianny, I. (2021). Phytochemical compounds and pharmacological activities of Vitis vinifera L: An updated review. Biointerface Research in Applied Chemistry, 11(5), 13829–13849.
Choi, H. J., Song, J. H., Park, K. S., and Baek, S. H. (2010). In vitro anti-enterovirus 71 activity of gallic acid from Woodfordia fruticosa flowers. Letters in Applied Microbiology, 50(4), 438–440. https://doi.org/10.1111/j.1472-765X.2010.02805.x
De Bona, K. S., Bonfanti, G., Bitencourt, P. E., da Silva, T. P., Borges, R. M., Boligon, A., Pigatto, A., Athayde, M. L., and Moretto, M. B. (2016). Protective effect of gallic acid and Syzygium cumini extract against oxidative stress-induced cellular injury in human lymphocytes. Drug and Chemical Toxicology, 39(3), 256–63. https://doi.org/10.3109/01480545.2015.1084631
Rashid, F., Javaid, A., Mahmood-Ur-Rahman, et al. (2022). Integrating pharmacological and computational approaches for the phytochemical analysis of <i>Syzygium cumini</i> and its anti-diabetic potential. Molecules (Basel, Switzerland), 27(17), 5734. https://doi.org/10.3390/molecules27175734
Ohifueme, A., Teralı, K., Olofinsan, K., Surgun, S., Ogbaga, C., and Ajiboye, T. (2019). Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. Journal of Food Biochemistry, 43, e12927. https://doi.org/10.1111/jfbc.12927
Nutan Modi, M., Goel, T., Das, T., Malik, S., Suri, S., Rawat, A. K., Srivastava, S. K., Tuli, R., Malhotra, S., and Gupta, S. K. (2013). Ellagic acid and gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease and reverse transcriptase activity. Indian Journal of Medical Research, 137(3), 540–8.
Reddivari, L., Vanamala, J., Safe, S. H., and Miller, J. C., Jr. (2010). The bioactive compounds alpha-chaconine and gallic acid in potato extracts decrease survival and induce apoptosis in LNCaP and PC3 prostate cancer cells. Nutrition and Cancer, 62(5), 601–10. https://doi.org/10.1080/01635580903532358
Li, L., Ng, T. B., Gao, W., Li, W., Fu, M., Niu, S. M., Zhao, L., Chen, R. R., and Fancy, Liu. (2005). Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice. Life Sciences, 77, 230–40. https://doi.org/10.1016/j.lfs.2004.12.024
BenSaad, L. A., Kim, K. H., Quah, C. C., et al. (2017). Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin AandB isolated from Punica granatum. BMC Complementary and Alternative Medicine, 17, 47. https://doi.org/10.1186/s12906-017-1555-0
Chatterjee, A., Chatterjee, S., Biswas, A., Bhattacharya, S., Chattopadhyay, S., and Bandyopadhyay, S. K. (2012). Gallic acid enriched fraction of Phyllanthus emblica potentiates indomethacin-induced gastric ulcer healing via e-NOS-dependent pathway. Evidence-Based Complementary and Alternative Medicine, 2012, 487380. https://doi.org/10.1155/2012/487380
García-Rivera, D., Delgado, R., Bougarne, N., Haegeman, G., and Berghe, W. V. (2011). Gallic acid indanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDA-MB231 breast cancer cells. Cancer Letters, 305(1), 21–31. https://doi.org/10.1016/j.canlet.2011.02.011
Selvaraj, J., Muthusamy, T., Srinivasan, C., and Balasubramanian, K. (2009). Impact of excess aldosterone on glucose homeostasis in adult male rat. Clinica Chimica Acta, 407(1–2), 51–57. https://doi.org/10.1016/j.cca.2009.06.030
Sancho, E., Cerón, J. J., and Ferrando, M. D. (2000). Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicology and Environmental Safety, 46(1), 81–86. https://doi.org/10.1006/eesa.1999.1888
Barcellos, L. J. G., Kreutz, L. C., Rodrigues, L. B., Fioreze, I., Quevedo, R. M., Cericato, L., Conrad, J., Soso, A. B., Fagundes, M., Lacerda, L. A., and Terra, S. (2003). Haematological and biochemical characteristics of male jundiá (Rhamdia Quelen, Quoy and GaimaRDT, Pimelodidae): Changes after acute stress. Aquaculture Research, 34, 1465–1469. https://doi.org/10.1111/j.1365-2109.2003.00972.x
Sharma, S. (2009). Tumor markers in clinical practice: General principles and guidelines. Indian Journal of Medical and Paediatric Oncology, 30(1), 1–8. https://doi.org/10.4103/0971-5851.56328
Ohkawa, H., Ohishi, N., and Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2), 351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Marklund, S., and Marklund, G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European journal of biochemistry, 47(3), 469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., and Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179(4073), 588–590. https://doi.org/10.1126/science.179.4073.588
Manjunathan, R., Devarajan, N., and Ragunathan, M. (2021). Possible mechanism of human recombinant leptin-induced VEGF A synthesis via PI3K/Akt/mTOR/S6 kinase signaling pathway while inducing angiogenesis: An analysis using chicken chorioallantoic membrane model. Journal of Vascular Research, 24, 1–18. https://doi.org/10.1159/000516498
Lopez, J., and Tait, S. W. (2015). Mitochondrial apoptosis: Killing cancer using the enemy within. British Journal of Cancer, 112(6), 957–962. https://doi.org/10.1038/bjc.2015.85
Hassan, M., Watari, H., AbuAlmaaty, A., Ohba, Y., and Sakuragi, N. (2014). Apoptosis and molecular targeting therapy in cancer. BioMed Research International, 2014, 150845. https://doi.org/10.1155/2014/150845
Arbiser, J. L., Bonner, M. Y., and Gilbert, L. C. (2017). Targeting the duality of cancer. NPJ Precision Oncology, 1, 23. https://doi.org/10.1038/s41698-017-0026-x
Chin, Y. W., Yoon, K. D., and Kim, J. (2009). Cytotoxic anticancer candidates from terrestrial plants. Anti-Cancer Agents in Medicinal Chemistry, 9(8), 913–942. https://doi.org/10.2174/187152009789124664
Oltvai, Z. N., Milliman, C. L., and Korsmeyer, S. J. (1993). Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell, 74(4), 609–619. https://doi.org/10.1016/0092-8674(93)90509-O
Shaikh, H., Bradhurst, P., Ma, L. X., Tan, S. Y. C., Egger, S. J., and Vardy, J. L. (2020). Body weight management in overweight and obese breast cancer survivors. Cochrane Database of Systematic Reviews, 12(12), CD12110. https://doi.org/10.1002/14651858.CD012110.pub2
Birgegård, G., Aapro, M. S., Bokemeyer, C., Dicato, M., Drings, P., Hornedo, J., Krzakowski, M., Ludwig, H., Pecorelli, S., Schmoll, H., Schneider, M., Schrijvers, D., Shasha, D., and Van Belle, S. (2020). Cancer-related anemia: Pathogenesis, prevalence and treatment. Oncology, 68(S1), 3–11. https://doi.org/10.1159/000083128
Murugesan, S., and Bhuavaneswari, S. (2012). Antitumor activity of Chondrococcus hornemanni and Spyridia fusiformis on Dalton’s lymphoma ascites in mice. Bangladesh Journal of Pharmacology, 7, 173–177. https://doi.org/10.3329/bjp.v7i3.11148
Kang, D. H. (2002). Oxidative stress, DNA damage, and breast cancer. AACN Clinical Issues, 13(4), 540–549. https://doi.org/10.1097/00044067-200211000-00007
Gomes Júnior, A. L., Paz, M. F., da Silva, L. I., Carvalho, S. D. A. C., Sobral, A. L., da Machado, K. C., Ferreira, P. M., Satyal, P., de Freitas, R. M., and Cavalcante, A. A. (2015). Serum oxidative stress markers and genotoxic profile induced by chemotherapy in patients with breast cancer A Pilot Study. Oxidative Medicine and Cellular Longevity, 2015, 212964. https://doi.org/10.1155/2015/212964
Rajneesh, C. P., Manimaran, A., Sasikala, K. R., and Adaikappan, P. (2008). Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Medical Journal, 49(8), 640–643.
Domaszewska, K., Janiak, A., Podgórski, T., Demuth, A., Kryściak, J., Perkowski, P., and Czerniak, U. (2021). A pilot study of influence of endurance training on the prooxidative and antioxidant status of women after breast cancer. International Journal of Environmental Research and Public Health, 18(6), 2822. https://doi.org/10.3390/ijerph18062822
Feng, Y., Spezia, M., Huang, S., Yuan, C., Zeng, Z., Zhang, L., Ji, X., Liu, W., Huang, B., Luo, W., Liu, B., Lei, Y., Du, S., Vuppalapati, A., Luu, H. H., Haydon, R. C., He, T. C., and Ren, G. (2018). Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes and Diseases, 5(2), 77–106. https://doi.org/10.1016/j.gendis.2018.05.001
Feng, M., Feng, C., Yu, Z., Fu, Q., Ma, Z., Wang, F., Wang, F., and Yu, L. (2015). Histopathological alterations during breast carcinogenesis in a rat model induced by 7,12-Dimethylbenz (a) anthracene and estrogen-progestogen combinations. International Journal of Clinical and Experimental Medicine, 8(1), 346–357.
Kaidoh, T., Yasugi, T., and Uehara, Y. (1991). The microvasculature of the 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat mammary tumour. I. Vascular patterns as visualized by scanning electron microscopy of corrosion casts. Virchows Archiv A, Pathological Anatomy and Histopathology, 418(2), 111–117. https://doi.org/10.1007/BF01600286
Gabe, V., Kacergius, T., Abu-Lafi, S., Zeidan, M., Abu-Farich, B., Austys, D., Masalha, M., and Rayan, A. (2019). Suppressive effects of octyl gallate on Streptococcus mutans biofilm formation, acidogenicity, and gene expression. Molecules, 24(17), 3170. https://doi.org/10.3390/molecules24173170
Acknowledgements
All the authors acknowledged the DBT-BIF Center, Holy Cross College, for providing the infrastructure facility to carry out the in silico analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vijayalakshmi, P., Indu, S., Ireen, C. et al. Octyl Gallate and Gallic Acid Isolated from Terminalia bellirica Circumvent Breast Cancer Progression by Enhancing the Intrinsic Apoptotic Signaling Pathway and Elevating the Levels of Anti-oxidant Enzymes. Appl Biochem Biotechnol 195, 7214–7235 (2023). https://doi.org/10.1007/s12010-023-04450-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04450-9