Abstract
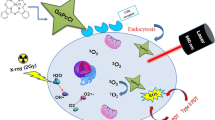
Breast cancer is the second most common cancer after lung cancer in the world. Due to the anti-cancer properties of Berberine (Ber), in this study, the effect of combination therapy of Ber in the presence of blue LED irradiation and Valproic acid (Val) on the MDA-MB-231 breast cancer cell line was investigated. For this reason, after culturing the cells using different concentrations of Ber and Val, breast cancer cells were treated in both mono-treatment and combination therapy. In combination therapy, two modes were considered: (1) treatment with Val and then treatment with Ber in the dark or in presence of blue light irradiation (PDT)at a wavelength of 465 nm and energy of 30 J/cm2 for 15 min, and (2) treatment with Ber in the dark or PDT and then treated with Val. In all cases, cell viability, morphological changes, and colonization were assessed. Evaluation of apoptosis was performed by fluorescence microscope and flow cytometry. According to the results, combination therapy has a higher mortality rate compared to mono-treatment, and in combination therapy, treatment of cells first with Ber (10 µg/mL)-PDT and then treatment with Val (250 µg/mL) caused a significant reduction (P < 0/05) in the survival rate of cancer cells. According to the findings, it can be said that the use of Ber-PDT in combination with Val, in addition to reducing the dose of the drug, has shown a synergistic effect which can suggest the potential of this strategy as a new treatment.








Similar content being viewed by others
References
Raigon Ponferrada, A., Guerrero Orriach, J. L., Molina Ruiz, J. C., Romero Molina, S., Gómez Luque, A., Cruz Mañas, J. (2021). Breast cancer and anaesthesia: Genetic influence. International Journal of Molecular Sciences [Internet]. 22(14):7653. Available from: https://www.mdpi.com/1422-0067/22/14/7653
Barzaman, K., Karami, J., Zarei, Z., Hosseinzadeh, A., Kazemi, M. H., Moradi-Kalbolandi, S. et al., (2020). Breast cancer: Biology, biomarkers, and treatments. International Immunopharmacology [Internet]. 84:106535. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1567576920304768
Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians [Internet]. 71(1):7–33. Available from: https://onlinelibrary.wiley.com/doi/10.3322/caac.21654
DeSantis, C. E., Ma, J., Gaudet, M. M., Newman, L. A., Miller, K. D., Goding Sauer, A. et al., (2019). Breast cancer statistics, 2019. CA: A Cancer Journal for Clinicians [Internet]. 69(6):438–51. Available from: https://onlinelibrary.wiley.com/doi/abs/10.3322/caac.21583
Chang-Qing, Y., Jie, L., Shi-Qi, Z., Kun, Z., Zi-Qian, G., Ran, X. et al., (2020). Recent treatment progress of triple-negative breast cancer. Progress in Biophysics and Molecular Biology [Internet]. 151:40–53. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0079610719301282
Garrido-Castro, A. C., Lin, N. U., Polyak, K. (2019) Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discovery [Internet]. 9(2):176–98. Available from: http://cancerdiscovery.aacrjournals.org/lookup/doi/10.1158/2159-8290.CD-18-1177
Won, K., & Spruck, C. (2020). Triple‑negative breast cancer therapy: Current and future perspectives (Review). International Journal of Oncology [Internet]. 57(6):1245–61. Available from: http://www.spandidos-publications.com/10.3892/ijo.2020.5135
Malhotra, M. K., & Emens, L. A. (2020). The evolving management of metastatic triple-negative breast cancer. Seminars Oncology [Internet]. 47(4):229–37. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0093775420300440
Bergin, A. R. T., & Loi, S. (2019). Triple-negative breast cancer: Recent treatment advances. F1000Research [Internet]. 8:1342. Available from: https://f1000research.com/articles/8-1342/v1
Aumeeruddy, M. Z., & Mahomoodally, M. F. (2019). Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer [Internet]. 125(10):1600–11. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cncr.32022
Lan, M., Zhao, S., Liu, W., Lee, C., Zhang, W., Wang, P. (2019). Photosensitizers for photodynamic therapy. Advanced Healthcare Materials [Internet]. 8(13):1900132. Available from: https://onlinelibrary.wiley.com/doi/10.1002/adhm.201900132
Nkune, N. W., Simelane, N. W. N., Montaseri, H., Abrahamse, H. (2021). Photodynamic therapy-mediated immune responses in three-dimensional tumor models. International Journal of Molecular Sciences [Internet]. 22(23):12618. Available from: https://www.mdpi.com/1422-0067/22/23/12618
Dobson, J., de Queiroz, G. F., Golding, J. P. (2018). Photodynamic therapy and diagnosis: Principles and comparative aspects. The Veterinary Journal [Internet]. 233:8–18. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1090023317302319
Oh, P.-S., Hwang, H., Jeong, H.-S., Kwon, J., Kim, H.-S., Kim, M. et al., (2016). Blue light-emitting diode induces apoptosis in lymphoid cells by stimulating autophagy. The International Journal of Biochemistry & Cell Biology [Internet]. 70:13–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1357272515300595
Yuan, Y., Yan, G., Gong, R., Zhang, L., Liu, T., Feng, C. et al., (2017). Effects of blue light emitting diode irradiation on the proliferation, apoptosis, and differentiation of bone marrow-derived mesenchymal stem cells. Cellular Physiology and Biochemistry [Internet]. 43(1):237–46. Available from: https://www.karger.com/Article/FullText/480344
Zhang, Q., & Li, L. (2018). Photodynamic combinational therapy in cancer treatment. Journal of B.U.ON., 23(3), 561–567
Loo, Y. S., Madheswaran, T., Rajendran, R., Bose, R. J. (2020). Encapsulation of berberine into liquid crystalline nanoparticles to enhance its solubility and anticancer activity in MCF7 human breast cancer cells. Journal of Drug Delivery Science and Technology [Internet]. 57:101756. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1773224720302628
Kumar, A., Ekavali., Chopra, K., Mukherjee, M., Pottabathini, R., Dhull, D. K. (2015). Current knowledge and pharmacological profile of berberine: An update. European Journal of Pharmacology [Internet]. 761:288–97. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0014299915300571
Zhang, C., Sheng, J., Li, G., Zhao, L., Wang, Y., Yang, W. et al., (2020). Effects of berberine and its derivatives on cancer: A systems pharmacology review. Frontiers in Pharmacology [Internet]. 10. Available from: https://www.frontiersin.org/article/10.3389/fphar.2019.01461/full
Tan, W., Li, Chen, M., Wang, Y. (2011). Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. International Journal of Nanomedicine [Internet]. 1773. Available from: http://www.dovepress.com/berberine-hydrochloride-anticancer-activity-and-nanoparticulate-delive-peer-reviewed-article-IJN
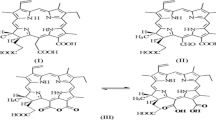
Cheng, L.-L., Wang, Y.-J., Huang, D.-H., Yao, S.-D., Ding, G.-J., Wang, S.-L., et al., (2014). Radiolysis and photolysis studies on active transient species of berberine. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy [Internet]. 124:670–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1386142514001061
Sajadpoor, Z., Amini-Farsani, Z., Teimori, H., Shamsara, M., Sangtarash, M. H., Ghasemi-Dehkordi, P., et al., (2018) Valproic acid promotes apoptosis and cisplatin sensitivity through downregulation of H19 noncoding RNA in ovarian A2780 cells. Applied Biochemistry and Biotechnology [Internet]. 185(4):1132–44. Available from: http://link.springer.com/10.1007/s12010-017-2684-0
Singh, D., Gupta, S., Verma, I., Morsy, M. A., Nair, A. B., Ahmed, A.-S. F. (2021). Hidden pharmacological activities of valproic acid: A new insight. Biomedicine & Pharmacotherapy [Internet]. 142:112021. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332221008040
Lipska, K., Gumieniczek, A., Filip A. A. (2020). Anticonvulsant valproic acid and other short-chain fatty acids as novel anticancer therapeutics: Possibilities and challenges. Acta Pharmaceutica [Internet]. 70(3):291–301. Available from: https://www.sciendo.com/article/10.2478/acph-2020-0021
Brodie, S. A., & Brandes, J. C. (2014). Could valproic acid be an effective anticancer agent? The evidence so far. Expert Review of Anticancer Therapy [Internet]. 14(10):1097–100. Available from: http://www.tandfonline.com/doi/full/10.1586/14737140.2014.940329
Heers, H., Stanislaw, J., Harrelson, J., Lee, M. W. (2018). Valproic acid is an adjunctive therapeutic agent for the treatment of breast cancer. European Journal of Pharmacology [Internet]. 835:61–74. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0014299918304308
Mokhtari, R. B., Homayouni, T. S., Baluch, N., Morgatskaya, E., Kumar, S., Das, B. et al., (2017). Combination therapy in combating cancer. Oncotarget [Internet]. 8(23):38022–43. Available from: https://www.oncotarget.com/lookup/doi/10.18632/oncotarget.16723
Hosseinzadeh, R., Khorsandi, K., Jahanshiri, M. (2017) Combination photodynamic therapy of human breast cancer using salicylic acid and methylene blue. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy [Internet]. 184:198–203. Available from: https://linkinghub.elsevier.com/retrieve/pii/S138614251730375X
Yin, N., Ma, W., Pei, J., Ouyang, Q., Tang, C., Lai, L. (2014). Synergistic and antagonistic drug combinations depend on network topology. Aloy P, editor. PLoS One [Internet]. 9(4):e93960. Available from: https://dx.plos.org/10.1371/journal.pone.0093960
Kasibhatla, S., Amarante-Mendes, G. P., Finucane, D., Brunner, T., Bossy-Wetzel, E., Green, D. R. (2006). Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb Protoc [Internet]. 2006(3):pdb.prot4493. Available from: http://www.cshprotocols.org/lookup/doi/10.1101/pdb.prot4493
Liang, Y., Zhang, H., Song, X., Yang, Q. (2020). Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Seminars in Cancer Biology [Internet]. 60:14–27. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1044579X1930063X
Nedeljković, M., & Damjanović, A. (2019). Mechanisms of chemotherapy resistance in triple-negative breast cancer—how we can rise to the challenge. Cells [Internet]. 22;8(9):957. Available from: https://www.mdpi.com/2073-4409/8/9/957
Ibrahim, T. S., Sheha, T. A., Abo-Dya, N. E., AlAwadh, M. A., Alhakamy, N. A., Abdel-Samii, Z. K. et al., (2020). Design, synthesis and anticancer activity of novel valproic acid conjugates with improved histone deacetylase (HDAC) inhibitory activity. Bioorganic Chemistry [Internet]. 99:103797. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0045206819322412
Zhu, M.-M., Li, H.-L., Shi, L.-H., Chen, X.-P., Luo, J., Zhang, Z.-L. (2017). The pharmacogenomics of valproic acid. Journal of Human Genetics [Internet]. 62(12):1009–14. Available from: http://www.nature.com/articles/jhg201791
Mawatari, T., Ninomiya, I., Inokuchi, M., Harada, S., Hayashi, H., Oyama, K., et al., (2015). Valproic acid inhibits proliferation of HER2-expressing breast cancer cells by inducing cell cycle arrest and apoptosis through Hsp70 acetylation. International Journal of Oncology [Internet]. 47(6):2073–81. Available from: https://www.spandidos-publications.com/10.3892/ijo.2015.3213
Li, G.-F., Qian, T.-L., Li, G.-S., Yang, C.-X., Qin, M., Huang, J., et al., (2012). Sodium valproate inhibits MDA-MB-231 breast cancer cell migration by upregulating NM23H1 expression. Genetics and Molecular Research [Internet]. 11(1):77–86. Available from: http://www.funpecrp.com.br/gmr/year2012/vol11-1/pdf/gmr1285.pdf
Ozman, Z., Ozbek Iptec, B., Sahin, E., Guney Eskiler, G., Deveci Ozkan, A., Kaleli, S. (2021). Regulation of valproic acid-induced EMT by AKT/GSK3β/β-catenin signaling pathway in triple-negative breast cancer. Molecular Biology Reports [Internet]. 48(2):1335–43. Available from: http://link.springer.com/10.1007/s11033-021-06173-8
Abdelkader, N. F., Elyamany, M., Gad, A. M., Assaf, N., Fawzy, H. M., Elesawy, W. H. (2020). Ellagic acid attenuates liver toxicity induced by valproic acid in rats. Journal of Pharmacological Sciences [Internet]. 143(1):23–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1347861320300086
Wawruszak, A., Halasa, M., Okon, E., Kukula-Koch, W., Stepulak, A. (2021). Valproic acid and breast cancer: State of the art in 2021. Cancers (Basel) [Internet]. 13(14):3409. Available from: https://www.mdpi.com/2072-6694/13/14/3409
Aghagolzade Haji, H., Khoshbin Khoshnazar, A. R., Gharaei, R., Javan, B., Asadi, J. (2014). Effect of valproic acid and radiotherapy on the viability of MCF-7 breast cancer cell line. Journal of Gorgan University of Medical Sciences [Internet]. [cited 2022 Feb 22];16(3):49–55. Available from: http://goums.ac.ir/journal/article-1-2120-en.html
Samadi, P., Sarvarian, P., Gholipour, E., Asenjan, K. S., Aghebati‐Maleki, L., Motavalli, R. et al., (2020) Berberine: A novel therapeutic strategy for cancer. IUBMB Life [Internet]. 72(10):2065–79. Available from: https://onlinelibrary.wiley.com/doi/10.1002/iub.2350
Xu, J., Long, Y., Ni, L., Yuan, X., Yu, N., Wu, R. et al., (2019). Anticancer effect of berberine based on experimental animal models of various cancers: A systematic review and meta-analysis. BMC Cancer [Internet]. 19(1):589. Available from: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-019-5791-1
El Khalki, L., Maire, V., Dubois, T., Zyad, A. (2020). Berberine impairs the survival of triple negative breast cancer cells: Cellular and molecular analyses. Molecules [Internet]. 25(3):506. Available from: https://www.mdpi.com/1420-3049/25/3/506
Gu, S., Song, X., Xie, R., Ouyang, C., Xie, L., Li, Q. et al., (2020). Berberine inhibits cancer cells growth by suppressing fatty acid synthesis and biogenesis of extracellular vesicles. Life Science [Internet]. 257:118122. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0024320520308730
Park, S. H., Sung, J. H., Kim, E. J., Chung, N. (2015). Berberine induces apoptosis via ROS generation in PANC-1 and MIA-PaCa2 pancreatic cell lines. Brazilian Journal of Medical and Biological Research [Internet]. 48(2):111–9. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0100-879X2015000200111&tlng=en
Lopes, T. Z., de Moraes, F. R., Tedesco, A. C., Arni, R. K., Rahal, P., Calmon, M. F. (2020). Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomedicine & Pharmacotherapy [Internet]. 123:109794. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0753332219354162
Yousefi Sadeghloo, A., Khorsandi, K., Kianmehr, Z. (2020). Synergistic effect of photodynamic treatment and doxorubicin on triple-negative breast cancer cells. Photochemical and Photobiological Sciences [Internet]. 19(11):1580–9. Available from: http://xlink.rsc.org/?DOI=D0PP00132E
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meschi, M., Khorsandi, K. & Kianmehr, Z. The Effect of Berberine Follow by Blue Light Irradiation and Valproic Acid on the Growth Inhibition of MDA-MB-231 Breast Cancer Cells. Appl Biochem Biotechnol 195, 6752–6767 (2023). https://doi.org/10.1007/s12010-023-04395-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04395-z