Abstract
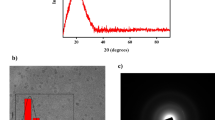
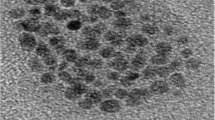
The use of cadmium sulfide nanoparticles (CdS-NPs) synthesized by fungi presents highly stable chemical and optical characteristics; this makes them a promising alternative for development of colorimetric methods for metal detection. Moreover, application of CdS-NPs is challenging due to the biological material used to carry out synthesis and coating is highly diverse; therefore, it is necessary to evaluate if such components are present in the biological material. Thus, the objective of this work was to detect metallic ions in synthetic water samples using CdS-NPs synthesized by the extract of Aspergillus niger. The conditions to produce fungal extracts were determined through a factorial design 23; additionally, biomolecules involved in metallic ions detection, synthesis, and coating of CdS-NPs were quantified; the studied biomolecules are NADH, sulfhydryl groups, proteins, and ferric reducing antioxidants (FRAP). CdS-NPs synthesized in this study were characterized by spectrophotometry, zeta potential, and high-resolution transmission electron microscopy (HRTEM). Finally, detection capacity of metallic ions in synthetic water samples was evaluated. It was proved that the methanolic extract of Aspergillus niger obtained under established conditions has the necessary components for both synthesis and coating of CdS-NPs, as well as detection of metallic ions because it was possible to synthesize CdS-NPs with a hexagonal crystalline structure with a length of 2.56 ± 0.50 nm which were able to detect Pb2+, Cr6+, and Fe3+ at pH 4 and Co2+ at pH 8.






Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Briffa, J., Sinagra, E., & Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Elsevier Ltd. https://doi.org/10.1016/j.heliyon.2020.e04691
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. In A. Luch (Ed.), Molecular, clinical and environmental toxicology. Experientia Supplementum (Vol. 101, pp. 133–164). Springer, Basel. https://doi.org/10.1007/978-3-7643-8340-4_6
Jin, M., Yuan, H., Liu, B., Peng, J., Xu, L., & Yang, D. (2020). Review of the distribution and detection methods of heavy metals in the environment. Analytical Methods, 12(48), 5747–5766. https://doi.org/10.1039/D0AY01577F
Odobašić, A., Šestan, I., & Begić, S. (2019). Biosensors for determination of heavy metals in waters. In Biosensors for Environmental Monitoring. IntechOpen. https://doi.org/10.5772/intechopen.84139
Govindasamy, M., Sriram, B., Wang, S. F., Chang, Y. J., & Rajabathar, J. R. (2020). Highly sensitive determination of cancer toxic mercury ions in biological and human sustenance samples based on green and robust synthesized stannic oxide nanoparticles decorated reduced graphene oxide sheets. Analytica Chimica Acta, 1137, 181–190. https://doi.org/10.1016/j.aca.2020.09.014
Baby, J. N., Sriram, B., Wang, S. F., George, M., Govindasamy, M., & Benadict Joseph, X. (2020). Deep eutectic solvent-based manganese molybdate nanosheets for sensitive and simultaneous detection of human lethal compounds: Comparing the electrochemical performances of M-molybdate (M = Mg, Fe, and Mn) electrocatalysts. Nanoscale, 12(38), 19719–19731. https://doi.org/10.1039/d0nr05533f
Aydin, Z., & Keleş, M. (2020). Colorimetric cadmium ion detection in aqueous solutions by newly synthesized Schiff bases. Turkish Journal of Chemistry, 44(3), 791. https://doi.org/10.3906/KIM-1912-36
Alorabi, A. Q., Abdelbaset, M., & Zabin, S. A. (2019). Colorimetric detection of multiple metal ions using Schiff base 1-(2-thiophenylimino)-4-(N-dimethyl)benzene. Chemosensors 2020, 8(1), 1. https://doi.org/10.3390/CHEMOSENSORS8010001
Reimann, M. J., Salmon, D. R., Horton, J. T., Gier, E. C., & Jefferies, L. R. (2019). Water-soluble sulfonate schiff-base ligands as fluorescent detectors for metal ions in drinking water and biological systems. ACS Omega, 4(2), 2874–2882. https://doi.org/10.1021/ACSOMEGA.8B02750/SUPPL_FILE/AO8B02750_SI_001.PDF
Chen, L., Tian, X., Xia, D., Nie, Y., Lu, L., Yang, C., & Zhou, Z. (2019). Novel colorimetric method for simultaneous detection and identification of multimetal ions in water: Sensitivity, selectivity, and recognition mechanism. ACS Omega, 4(3), 5915–5922. https://doi.org/10.1021/ACSOMEGA.9B00312/SUPPL_FILE/AO9B00312_SI_001.PDF
Abed, T., Kadem, B., & Kadhim, R. (2019). Simple colorimetric method using aqueous solution to detect heavy metal | Iraqi Journal of Science. Iraqi Journal of Science, 60, 28–33. Retrieved from https://ijs.uobaghdad.edu.iq/index.php/eijs/article/view/709
Lou, Y., Zhao, Y., Chen, J., & Zhu, J. J. (2014). Metal ions optical sensing by semiconductor quantum dots. Journal of Materials Chemistry C, 2(4), 595–613. https://doi.org/10.1039/c3tc31937g
Sharma, G., Pandey, S., Ghatak, S., Watal, G., & Rai, P. K. (2018). Potential of spectroscopic techniques in the characterization of “ green nanomaterials". nanomaterials in plants, algae, and microorganisms. Elsevier Inc. https://doi.org/10.1016/B978-0-12-811487-2.00003-7
Durán, N., Marcato, P. D., Durán, M., Yadav, A., Gade, A., & Rai, M. (2011). Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Applied Microbiology and Biotechnology, 90(5), 1609–1624. https://doi.org/10.1007/s00253-011-3249-8
Khandel, P., & Shahi, S. K. (2018). Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. Journal of Nanostructure in Chemistry, 8(4), 369–391. https://doi.org/10.1007/s40097-018-0285-2
Priyanka, U., Akshay Gowda, K. M., Elisha, M. G., Surya Teja, B., Nitish, N., & Raj Mohan, B. (2016). Biologically synthesized PbS nanoparticles for the detection of arsenic in water. International Biodeterioration & Biodegradation, 1–9. https://doi.org/10.1016/j.ibiod.2016.10.009
Uddandarao, P., & Balakrishnan, R. M. (2017). Thermal and optical characterization of biologically synthesized ZnS nanoparticles synthesized from an endophytic fungus Aspergillus flavus: A colorimetric probe in metal detection. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy, 175, 200–207. https://doi.org/10.1016/j.saa.2016.12.021
Uddandarao, P., & Mohan, R. (2016). ZnS semiconductor quantum dots production by an endophytic fungus Aspergillus flavus. Materials Science and Engineering B: Solid-State Materials for Advanced Technology, 207, 26–32. https://doi.org/10.1016/j.mseb.2016.01.013
Sandoval-Cárdenas, I., Gómez-Ramírez, M., & Rojas-Avelizapa, N. G. (2017). Use of a sulfur waste for biosynthesis of cadmium sulfide quantum dots with Fusarium oxysporum f. sp. lycopersici. Materials Science in Semiconductor Processing, 63, 33–39. https://doi.org/10.1016/j.mssp.2017.01.017
Hussain, I., Singh, N. B., Singh, A., Singh, H., & Singh, S. C. (2016). Green synthesis of nanoparticles and its potential application. Biotechnology Letters, 38(4), 545–560. https://doi.org/10.1007/S10529-015-2026-7
Ahmad, T., Wani, I. A., Manzoor, N., Ahmed, J., & Asiri, A. M. (2013). Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids and Surfaces B: Biointerfaces, 107, 227–234. https://doi.org/10.1016/j.colsurfb.2013.02.004
Chowdhury, S., Basu, A., & Kundu, S. (2014). Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Research Letters, 9(1), 1–11. https://doi.org/10.1186/1556-276X-9-365
Kobashigawa, J. M., Robles, C. A., Martínez Ricci, M. L., & Carmarán, C. C. (2019). Influence of strong bases on the synthesis of silver nanoparticles (AgNPs) using the ligninolytic fungi Trametes trogii. Saudi Journal of Biological Sciences, 26(7), 1331–1337. https://doi.org/10.1016/j.sjbs.2018.09.006
Shaligram, N., Bule, M., Bhambure, R., Singhal, R., Singh, S. K., Szakacs, G., & Pandey, A. (2009). Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochemistry, 44(8), 939–943. https://doi.org/10.1016/j.procbio.2009.04.009
Ahmad, A., Mukherjee, P., Mandal, D., Senapati, S., Khan, M. I., Kumar, R., & Sastry, M. (2002). Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. Journal of the American Chemical Society, 124(41), 12108–12109. https://doi.org/10.1021/ja027296o
Reyes, L. R., Gómez, I., & Garza, M. T. (2009). Biosynthesis of cadmium sulfide nanoparticles by the fungi Fusarium sp. International Journal of Green Nanotechnology: Biomedicine, 1(1), 90–95. https://doi.org/10.1080/19430850903149936
Bel Haj, M., Ben Brahim, N., Mrad, R., Haouari, M., Ben Chaâbane, R., & Negrerie, M. (2018). Use of MPA-capped CdS quantum dots for sensitive detection and quantification of Co2+ ions in aqueous solution. Analytica Chimica Acta, 1028, 50–58. https://doi.org/10.1016/j.aca.2018.04.041
Giancaspero, T. A., Locato, V., & Barile, M. (2013). A regulatory role of NAD redox status on flavin cofactor homeostasis in S . cerevisiae Mitochondria. Oxidative Medicine and Cellular Longevity, 2013, 16. https://doi.org/10.1155/2013/612784
Vijayalakshmi, M., & Ruckmani, K. (2016). Ferric reducing anti-oxidant power assay in plant extract. Bangladesh Journal of Pharmacology, 11(3), 570–572. https://doi.org/10.3329/bjp.v11i3.27663
Beveridge, T., Toma, S., & Nakai, S. (1974). Determination of SH- and SS-groups in some food proteins using Ellman ’s reagent. New York, 39, 49–51. Retrieved from https://doi.org/10.1111/j.1365-2621.1974.tb00984.x
Suresh, S. (2013). Semiconductor nanomaterials, methods and applications: A review. Nanoscience and Nanotechnology, 3(3), 62–74. https://doi.org/10.5923/j.nn.20130303.06
Valu, M. V., Soare, L. C., Sutan, N. A., Ducu, C., Moga, S., Hritcu, L., … Carradori, S. (2020). Optimization of ultrasonic extraction to obtain erinacine a and polyphenols with antioxidant activity from the fungal biomass of Hericium erinaceus. Foods, 9(12), 1–17. https://doi.org/10.3390/foods9121889
Cetin, B. M., Ceylan, G., Cantürk, Z., Öztürk, N., Babayiğit, M. K., Yüzüak, S., & Yamaç, M. (2021). Screening of antioxidant activity of mycelia and culture liquids of fungi from Turkey. Microbiology (Russian Federation), 90(1), 133–143. https://doi.org/10.1134/S0026261721010033
Jernejc, K. (2004). Comparison of different methods for metabolite extraction from Aspergillus niger mycelium. Acta Chimica Slovenica, 51(3), 567–578.
Zekri, N., Zerkani, H., Elazzouzi, H., Zair, T., & El Belghiti, M. A. (2021). Extracts of m. pulegium (l.) and m. spicata (l.): effect of extraction conditions on phenolics and flavonoids contents and their antioxidant power. Egyptian Journal of Chemistry, 64(3), 1447–1459. https://doi.org/10.21608/EJCHEM.2020.43922.2893
Yang, L., Lübeck, M., & Lübeck, P. S. (2017). Aspergillus as a versatile cell factory for organic acid production. Fungal Biology Reviews, 31(1), 33–49. https://doi.org/10.1016/J.FBR.2016.11.001
Yu, R., Liu, J., Wang, Y., Wang, H., & Zhang, H. (2021). Aspergillus niger as a secondary metabolite factory. Frontiers in Chemistry, 9, 434. https://doi.org/10.3389/FCHEM.2021.701022/BIBTEX
Carvalho, C. R. De, Ferreira, M. C., Amorim, S. S., Hellen, R., Catarine, J., Assis, S. De, … Rosa, L. H. (2019). Recent advancement in white biotechnology through fungi (Vol. 3). Retrieved from http://link.springer.com/10.1007/978-3-030-10480-1
Nakilcioğlu-Taş, E., & Ötleş, S. (2021). Influence of extraction solvents on the polyphenol contents, compositions, and antioxidant capacities of fig(Ficus carica l.) seeds. Anais da Academia Brasileira de Ciencias, 93(1), 1–11. https://doi.org/10.1590/0001-3765202120190526
Hietzschold, S., Walter, A., Davis, C., Taylor, A. A., & Sepunaru, L. (2019). Does nitrate reductase play a role in silver nanoparticle synthesis? Evidence for NADPH as the sole reducing agent. ACS Sustainable Chemistry and Engineering, 7(9), 8070–8076. https://doi.org/10.1021/acssuschemeng.9b00506. rapid-communication.
Kumar, S. A., Abyaneh, M. K., Gosavi, S. W., Kulkarni, S. K., Pasricha, R., Ahmad, A., & Khan, M. I. (2007). Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO 3. Biotechnology Letters, 29(3), 439–445. https://doi.org/10.1007/s10529-006-9256-7
Talekar, S., Joshi, A., Chougle, R., Nakhe, A., & Bhojwani, R. (2016). Immobilized enzyme mediated synthesis of silver nanoparticles using cross-linked enzyme aggregates (CLEAs) of NADH-dependent nitrate reductase. Nano-Structures and Nano-Objects, 6, 23–33. https://doi.org/10.1016/j.nanoso.2016.03.002
Mahendran, G., & Ranjitha, B. D. (2016). Biological activities of silver nanoparticles from Nothapodytes nimmoniana (Graham)Mabb. fruit extracts. Food Science and Human Wellness, 5(4), 207–218. https://doi.org/10.1016/j.fshw.2016.10.001
Phongtongpasuk, S., Poadang, S., & Yongvanich, N. (2016). Environmental-friendly method for synthesis of silver nanoparticles from dragon fruit peel extract and their antibacterial activities. Energy Procedia, 89, 239–247. https://doi.org/10.1016/j.egypro.2016.05.031
Salari, S., Esmaeilzadeh, S., & Samzadeh-kermani, A. (2018). In-vitro evaluation of antioxidant and antibacterial potential of green synthesized silver nanoparticles using Prosopis farcta fruit extract. Iranian Journal of Pharmaceutical Research, 18(1), 430–445. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6487442/pdf/ijpr-18-430.pdf
Abolhasani, J., Hassanzadeh, J., & Jalali, E. S. (2014). Ultrasensitive determination of lead and chromium contamination in well and dam water based on fluorescence quenching of CdS quantum dots. International Nano Letters, 4(4), 65–72. https://doi.org/10.1007/s40089-014-0120-9
Chen, J., Zheng, A. F., Gao, Y., He, C., Wu, G., Chen, Y., … Zhu, C. (2008). Functionalized CdS quantum dots-based luminescence probe for detection of heavy and transition metal ions in aqueous solution. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy, 69(3), 1044–1052. https://doi.org/10.1016/j.saa.2007.06.021
Chen, Y., & Rosenzweig, Z. (2002). Luminescent CdS quantum dots as selective ion probes. Analytical Chemistry, 74(19), 5132–5138. https://doi.org/10.1021/ac0258251
Gallagher, S. A., Moloney, M. P., Wojdyla, M., Quinn, S. J., Kelly, J. M., & Gun’Ko, Y. K. (2010). Synthesis and spectroscopic studies of chiral CdSe quantum dots. Journal of Materials Chemistry, 20(38), 8350–8355. https://doi.org/10.1039/c0jm01185a
Jiao, H., Zhang, L., Liang, Z., Peng, G., & Lin, H. (2014). Size-controlled sensitivity and selectivity for the fluorometric detection of Ag+ by homocysteine capped CdTe quantum dots. Microchimica Acta, 181(11–12), 1393–1399. https://doi.org/10.1007/s00604-014-1276-8
Koneswaran, M., & Narayanaswamy, R. (2009). l-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sensors and Actuators, B: Chemical, 139(1), 104–109. https://doi.org/10.1016/j.snb.2008.09.028
Naik, R. R., Stringer, S. J., Agarwal, G., Jones, S. E., & Stone, M. O. (2002). Biomimetic synthesis and patterning of silver nanoparticles. Nature Materials, 1(3), 169–172. https://doi.org/10.1038/nmat758
Wei, F., Yu, H., Hu, M., Xu, G., Cai, Z., Yang, J., … Hu, Q. (2012). Manganese modified CdTe/CdS quantum dots as a highly selective probe to detect trace copper element in beer samples. Analytical Methods, 4(5), 1438–1444. https://doi.org/10.1039/c2ay05794h
Kelly, R. T., & Love, N. G. (2007). Ultraviolet spectrophotometric determination of nitrate: Detecting nitrification rates and inhibition. Water Environment Research, 79(7), 808–812. https://doi.org/10.2175/106143007x156682
Raynal, B., Lenormand, P., Baron, B., Hoos, S., & England, P. (2010). Quality assessment and optimization of purified protein samples: why and how? Microbial Cell Factories, 13(1). https://doi.org/10.1186/s12934-014-0180-6
Taniguchi, M., & Lindsey, J. S. (2018). Database of absorption and fluorescence spectra of >300 common compounds for use in PhotochemCAD. Photochemistry and Photobiology, 94(2), 290–327. https://doi.org/10.1111/php.12860
Alsaggaf, M. S., Elbaz, A. F., Badawy, S. E., & Moussa, S. H. (2020). Anticancer and antibacterial activity of cadmium sulfide nanoparticles by Aspergillus niger. Advances in Polymer Technology, 2020, 1–13. https://doi.org/10.1155/2020/4909054
Loa, J. D. A., Castellanos-Angeles, A., García-Tejeda, L. Á., Rivas-Castillo, A. M., & Rojas-Avelizapa, N. G. (2021). Assessment of cadmium sulfide nanoparticles synthesis by cadmium-tolerant fungi. Lecture Notes in Networks and Systems, 297, 145–156. https://doi.org/10.1007/978-3-030-82064-0_12
Coello, V. (2019). Nanofotónica. Los grandes avances y retos de un mundo pequeño. Mundo Nano. Revista Interdisciplinaria en Nanociencias y Nanotecnología, 13(24), e1–e4. https://doi.org/10.22201/ceiich.24485691e.2020.24.69607
Perucho, J., Gonzalo-Gobernado, R., Bazan, E., Casarejos, M. J., Jiménez-Escrig, A., Asensio, M. J., & Herranz, A. S. (2015). Optimal excitation and emission wavelengths to analyze amino acids and optimize neurotransmitters quantification using precolumn OPA-derivatization by HPLC. Amino Acids, 47(5), 963–973. https://doi.org/10.1007/s00726-015-1925-1
Gadalla, A., Almokhtar, M., & Abouelkhir, A. N. (2018). Effect of Mn doping on structural, optical and magnetic properties of CdS diluted magnetic semiconductor nanoparticles. Chalcogenide Letters, 15(4), 207–218.
Khan, A. (2012). CdS nanoparticles with a thermoresponsive polymer: Synthesis and properties. Journal of Nanomaterials, 2012. https://doi.org/10.1155/2012/451506
Kozhevnikova, N. S., Vorokh, A. S., & Uritskaya, A. A. (2015). Cadmium sulfide nanoparticles prepared by chemical bath deposition. Russian Chemical Reviews, 84(3), 225–250. https://doi.org/10.1070/rcr4452
Su, J., Zhang, T., Li, Y., Chen, Y., & Liu, M. (2016). Photocatalytic activities of copper doped cadmium sulfide microspheres prepared by a facile ultrasonic spray-pyrolysis method. Molecules, 21(6), 735. https://doi.org/10.3390/molecules21060735
Qu, C., Yang, S., Mortimer, M., Zhang, M., Chen, J., Wu, Y., … Huang, Q. (2022). Functional group diversity for the adsorption of lead(Pb) to bacterial cells and extracellular polymeric substances. Environmental Pollution, 295, 118651. https://doi.org/10.1016/J.ENVPOL.2021.118651
Atieh, M. A., Bakather, O. Y., Al-Tawbini, B., Bukhari, A. A., Abuilaiwi, F. A., & Fettouhi, M. B. (2010). Effect of carboxylic functional group functionalized on carbon nanotubes surface on the removal of lead from water. Bioinorganic Chemistry and Applications, 2010. https://doi.org/10.1155/2010/603978
Pietrzyk, D. J., & Frank, C. W. (1979). Oxidizing and reducing agents as titrants in analytical chemistry. In D. Pietrzyk & C. W. Frank (Eds.), Analytical chemistry (pp. 265–290). Academic Press. https://doi.org/10.1016/B978-0-12-555160-1.50016-2
Christensen, L., & Stulc, D. (1979). Reacciones químicas que afectan la filtrabilidad en el acondicionamiento de lodos de hierro y cal en JSTOR. Journal (Water Pollution Control Federation), 51, 499–512. Retrieved from http://www.jstor.org/stable/25040453
Howell, O. R., & Jackson, A. (1933). The change in the absorption spectrum of cobalt chloride in aqueous solution with increasing concentration of hydrochloric acid. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, 142(847), 587–597. https://doi.org/10.1098/RSPA.1933.0190
Acknowledgements
The author thanks the Biochemical Engineer Ma. Lourdes Palma Tirado for her support with the transmission electron microscopy analysis.
Funding
The project was funded by Instituto Politécnico Nacional, Consejo Nacional de Ciencia y Tecnología (CONACyT) by the project A1-S-31777.
Author information
Authors and Affiliations
Contributions
J.D.A. Loa: investigation, methodology, writing, editing, formal analysis; I.A. Cruz-Rodríguez: writing, review, visualization; N. G. Rojas-Avelizapa: conceptualization, validation, review, editing, visualization, supervision, project administration.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable. The research did not involve human participants.
Consent to Publish
Not applicable. The research did not involve human participants.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loa, J.D.A., Cruz-Rodríguez, I.A. & Rojas-Avelizapa, N.G. Colorimetric Detection of Metals Using CdS-NPs Synthesized by an Organic Extract of Aspergillus niger. Appl Biochem Biotechnol 195, 4148–4163 (2023). https://doi.org/10.1007/s12010-023-04341-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04341-z