Abstract
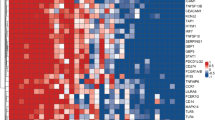
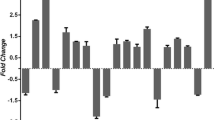
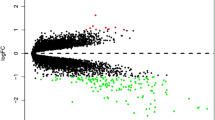
Mycobacterium tuberculosis (M.tb) could induce type IV hypersensitivity. The chemotaxis of the leukocytes toward the site of infection and producing matrix metalloproteinases (MMPs) are key factors in the immune pathogenesis of tuberculosis (TB). Mononuclear cells were isolated from bronchoalveolar lavage (BAL) specimens, and the target from genomic DNA was used for qPCR TB diagnosis and cDNA for specific RT-qPCR gene expression. The subjects were then classified into TB+ and TB− groups, and the expression levels of CFP-10, ESAT-6, CCR1, CCR12 and MMP3,9 were evaluated. The mean level of CCR1 expression in TB+ and TB− patients’ BAL was 1.71 ± 0.78 and 0.5 ± 0.22, respectively, which was statistically different (p = 0.01). The CCR2 level, in TB+ (2.07 ± 1.4), was higher than in TB− patients (1.42 ± 0.89, p = 0.01). The MMP9 expression in TB+ was 2.56 ± 0.68, also higher than in TB− patients (1.13 ± 0.35), while MMP3 was lower in TB+ (0.22 ± 0.09) than in TB− (0.64 ± 0.230, p = 0.05). The CCR2/CCR1 and MMP3/MMP9 balance in TB+ were reduced, compared to the TB−. The CFP-10 and ESAT-6 were highly expressed in TB+ patients. The CFP-10 expression had a strong negative correlation with albumin (r = − 0.93, p = 0.001), and a negative correlation with neutrophil (r = − 0.444, p = 0.1 with 90% CI). The MMP-9 expression showed a positive correlation with WBC count (r = 0.61, p = 0.02), in TB+, and had a negative correlation with BMI (r = 0.59, p = 0.02) in TB−. The M.tb CFP-10 might be implicated in lowering CCR2 and MMP3 expression in favour of M.tb dissemination. Moreover, the balance of CCR2/CCR1 and MMP3/MMP9 can be used as prognostic factors in the severity of TB.
Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this manuscript and are available from the corresponding author upon reasonable request.
Abbreviations
- APCs:
-
Antigen-presenting cells
- BAL:
-
Bronchoalveolar lavage
- CCR1:
-
C-C chemokine receptor type 1
- CCR2:
-
C-C chemokine receptor type 1
- CFP-10:
-
Culture filtrate protein 10 kDa
- ESAT-6:
-
Early secretory antigenic target 6 kDa
- M.tb :
-
Mycobacterium tuberculosis
- MMP:
-
Matrix metalloproteinases
- PBMCs:
-
Peripheral blood mononuclear cells
- qPCR:
-
Real-time PCR
- RT-qPCR:
-
Reverse transcription qPCR
- TB:
-
Tuberculosis
References
Sulis, G., Roggi, A., Matteelli, A., & Raviglione, M. C. (2014). Tuberculosis: epidemiology and control. Mediterranean Journal of Hematology and Infectious Diseases, 6(1), e2014070. https://doi.org/10.4084/mjhid.2014.070
Boggiano, C., Eichelberg, K., Ramachandra, L., Shea, J., Ramakrishnan, L., Behar, S., Ernst, J. D., Porcelli, S. A., Maeurer, M., & Kornfeld, H. (2017). The impact of Mycobacterium tuberculosis Immune evasion on protective immunity: Implications for TB vaccine design—meeting report. Vaccine, 35(27), 3433–3440. https://doi.org/10.1016/j.vaccine.2017.04.007
Singh, A., Prasad, R., Balasubramanian, V., & Gupta, N. (2020). Drug-resistant tuberculosis and HIV infection: Current perspectives. HIV AIDS (Auckl). 12, 9–31. https://doi.org/10.2147/HIV.S193059
Li, W., Deng, G., Li, M., Zeng, J., Zhao, L., Liu, X., & Wang, Y. (2014). A recombinant adenovirus expressing CFP10, ESAT6, Ag85A and Ag85B of Mycobacterium tuberculosis elicits strong antigen-specific immune responses in mice. Molecular Immunology, 62(1), 86–95. https://doi.org/10.1016/j.molimm.2014.06.007
Zhai, W., Wu, F., Zhang, Y., Fu, Y., & Liu, Z. (2019). The immune escape mechanisms of Mycobacterium tuberculosis. International Journal of Molecular Sciences, 20(2), 340. https://doi.org/10.3390/ijms20020340
Monin, L., & Khader, S. A. (2014). Chemokines in tuberculosis: The good, the bad and the ugly. Seminars in Immunology, 26(6), 552–558. https://doi.org/10.1016/j.smim.2014.09.004
Domingo-Gonzalez, R., Prince, O., Cooper, A., & Khader, S. A. (2016). Cytokines and chemokines in Mycobacterium tuberculosis infection. Microbiol Spectr 4(5):10.1128/microbiolspec.TBTB2-0018-2016. https://doi.org/10.1128/microbiolspec.TBTB2-0018-2016
Samstein, M., Schreiber, H. A., Leiner, I. M., Susac, B., Glickman, M. S., & Pamer, E. G. (2013). Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. Elife, 2, e01086. https://doi.org/10.7554/eLife.01086
Tiwari, S., Casey, R., Goulding, C. W., Hingley-Wilson, S., Jacobs, W. R. Jr. (2019). Infect and inject: How Mycobacterium tuberculosis exploits its major virulence-associated type VII secretion system, ESX-1. Microbiology Spectrum, 7(3), 10.1128/microbiolspec.BAI-0024-2019. https://doi.org/10.1128/microbiolspec.BAI-0024-2019.
Abebe, F., Belay, M., Legesse, M., Mihret, A., & Franken, K. S. (2017). Association of ESAT-6/CFP-10-induced IFN-γ, TNF-α and IL-10 with clinical tuberculosis: Evidence from cohorts of pulmonary tuberculosis patients, household contacts and community controls in an endemic setting. Clinical and Experimental Immunology, 189(2), 241–249. https://doi.org/10.1111/cei.12972
Brew, K., Dinakarpandian, D., & Nagase, H. (2000). Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochimica et Biophysica Acta, 1477(1–2), 267–283. https://doi.org/10.1016/s0167-4838(99)00279-4
Ong, C. W., Elkington, P. T., & Friedland, J. S. (2014). Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. American Journal of Respiratory and Critical Care Medicine, 190(1), 9–18. https://doi.org/10.1164/rccm.201311-2106PP
Quiding-Jarbrink, M., Smith, D. A., & Bancroft, G. J. (2001). Production of matrix metalloproteinases in response to mycobacterial infection. Infection and Immunity, 69(9), 5661–5670. https://doi.org/10.1128/iai.69.9.5661-5670.2001
Rivera-Marrero, C. A., Schuyler, W., Roser, S., Ritzenthaler, J. D., Newburn, S. A., & Roman, J. (2002). M. tuberculosis induction of matrix metalloproteinase-9: the role of mannose and receptor-mediated mechanisms. American Journal of Physiology Lung Cellular and Molecular Physiology, 282(3), L546-555. https://doi.org/10.1152/ajplung.00175.2001
Ragno, S., Romano, M., Howell, S., Pappin, D. J., Jenner, P. J., & Colston, M. J. (2001). Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: A combined transcriptomic and proteomic approach. Immunology, 104(1), 99–108. https://doi.org/10.1046/j.0019-2805.2001.01274.x
Torrado, E., Fountain, J. J., Liao, M., Tighe, M., Reiley, W. W., Lai, R. P., Meintjes, G., Pearl, J. E., Chen, X., Zak, D. E., Thompson, E. G., Aderem, A., Ghilardi, N., Solache, A., McKinstry, K. K., Strutt, T. M., Wilkinson, R. J., Swain, S. L., & Cooper, A. M. (2015). Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. Journal of Experimental Medicine, 212(9), 1449–1463. https://doi.org/10.1084/jem.20141520
Riahi, F., Derakhshan, M., Mosavat, A., Soleimanpour, S., & Rezaee, S. A. (2015). Evaluation of point mutation detection in Mycobacterium tuberculosis with isoniazid resistance using real-time PCR and TaqMan probe assay. Applied Biochemistry and Biotechnology, 175(5), 2447–2455. https://doi.org/10.1007/s12010-014-1442-9
Hunter, RL. (2020) The pathogenesis of tuberculosis—the Koch phenomenon reinstated. Pathogens, 9(10). https://doi.org/10.3390/pathogens9100813
Kumar, R., Singh, P., Kolloli, A., Shi, L., Bushkin, Y., Tyagi, S., & Subbian, S. (2019). Immunometabolism of phagocytes during Mycobacterium tuberculosis infection. Frontiers in Molecular Biosciences, 6, 105. https://doi.org/10.3389/fmolb.2019.00105
Slight, S. R., & Khader, S. A. (2013). Chemokines shape the immune responses to tuberculosis. Cytokine & Growth Factor Reviews, 24(2), 105–113. https://doi.org/10.1016/j.cytogfr.2012.10.002
Pokkali, S., Das, S. D., & L, R. (2008). Expression of CXC and CC type of chemokines and its receptors in tuberculous and non-tuberculous effusions. Cytokine, 41(3), 307–314. https://doi.org/10.1016/j.cyto.2007.12.009
Sadek, M. I., Sada, E., Toossi, Z., Schwander, S. K., & Rich, E. A. (1998). Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. American Journal of Respiratory Cell and Molecular Biology, 19(3), 513–521. https://doi.org/10.1165/ajrcmb.19.3.2815
Loetscher, P., Seitz, M., Baggiolini, M., & Moser, B. (1996). Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. Journal of Experimental Medicine, 184(2), 569–577.
Romero, I. A., Prevost, M. C., Perret, E., Adamson, P., Greenwood, J., Couraud, P. O., & Ozden, S. (2000). Interactions between brain endothelial cells and human T-cell leukaemia virus type 1-infected lymphocytes: Mechanisms of viral entry into the central nervous system. Journal of Virology, 74(13), 6021–6030. https://doi.org/10.1128/jvi.74.13.6021-6030.2000
Zhang, C. (2008). The role of inflammatory cytokines in endothelial dysfunction. Basic Research in Cardiology, 103(5), 398–406. https://doi.org/10.1007/s00395-008-0733-0
Abebe, F., Holm-Hansen, C., Wiker, H. G., & Bjune, G. (2007). Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scandinavian Journal of Immunology, 66(2–3), 176–191. https://doi.org/10.1111/j.1365-3083.2007.01978.x
Scott, H. M., & Flynn, J. L. (2002). Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: Influence of dose on disease progression. Infection and Immunity, 70(11), 5946–5954. https://doi.org/10.1128/iai.70.11.5946-5954.2002
Lyadova, I. V., & Panteleev, A. V. (2015). Th1 and Th17 Cells in tuberculosis: protection, pathology, and biomarkers. Mediators of Inflammation, 2015, 854507. https://doi.org/10.1155/2015/854507
Dwivedi, V. P., Bhattacharya, D., Chatterjee, S., Prasad, D. V., Chattopadhyay, D., Van Kaer, L., Bishai, W. R., & Das, G. (2012). Mycobacterium tuberculosis directs T helper 2 cell differentiation by inducing interleukin-1β production in dendritic cells. Journal of Biological Chemistry, 287(40), 33656–33663. https://doi.org/10.1074/jbc.M112.375154
Natarajan, K., Latchumanan, V. K., Singh, B., Singh, S., & Sharma, P. (2003). Down-regulation of T helper 1 responses to mycobacterial antigens due to maturation of dendritic cells by 10-kDa mycobacterium tuberculosis secretory antigen. Journal of Infectious Diseases, 187(6), 914–928. https://doi.org/10.1086/368173
Trajkovic, V., Natarajan, K., & Sharma, P. (2004). Immunomodulatory action of mycobacterial secretory proteins. Microbes and Infection, 6(5), 513–519. https://doi.org/10.1016/j.micinf.2003.12.015
Muefong, C. N., & Sutherland, J. S. (2020). Neutrophils in tuberculosis-associated inflammation and lung pathology. Frontiers in Immunology, 11, 962. https://doi.org/10.3389/fimmu.2020.00962
Hilda, J. N., Das, S., Tripathy, S. P., & Hanna, L. E. (2020). Role of neutrophils in tuberculosis: A bird’s eye view. Innate Immunity, 26(4), 240–247. https://doi.org/10.1177/1753425919881176
Sharebiani, H., Hajimiri, S., Abbasnia, S., Soleimanpour, S., Hashem Asnaashari, A. M., Valizadeh, N., Derakhshan, M., Pilpa, R., Firouzeh, A., Ghazvini, K., AmelJamehdar, S., & Rezaee, S. A. (2021). Game theory applications in host-microbe interactions toward disease manifestation: Mycobacterium tuberculosis infection as an example. Iranian Journal of Basic Medical Sciences, 24(10), 1324–1335. https://doi.org/10.22038/ijbms.2021.55471.12410
Sharebiani, H., Abbasnia, S., Soleimanpour, S., & Rezaee, S. A. R. (2020). Host-microbe interactions in manifestation of tuberculosis: A system biology study in implicated compartments. bioRxiv 2020.12.06.413617. https://doi.org/10.1101/2020.12.06.413617
Funding
This study was subjected to an MSc thesis in Medical Microbiology and financially supported by the Vice-Chancellor for Research and Technology, Mashhad University of Medical Sciences, Mashhad, Iran, under grants MUMS. 930690 and MUMS. 941165.
Author information
Authors and Affiliations
Contributions
SA, SH and FM collected the samples and performed the experiments. HA, as a pulmonologist, monitored the patients and took the BAL. NA, SMJ and AM compiled the data and prepared the draft. MD and SAJ were the co-supervisors. KG and SAR supervised the study. SAR planned the study, did the statistics and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Research Involving Human Participants and Informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was reviewed, approved and supervised by the Biomedical Research Ethics Committee of the Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.REC.930690 and IR.MUMS.REC. 941165), and written informed consent forms were obtained and signed by all participants.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbasnia, S., Hajimiri, S., Jafari Rad, M. et al. Gene Expression Study of Host and Mycobacterium tuberculosis Interactions in the Manifestation of Acute Tuberculosis. Appl Biochem Biotechnol 195, 3641–3652 (2023). https://doi.org/10.1007/s12010-023-04329-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04329-9