Abstract
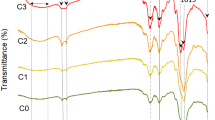
The fabrication of multifunctional scaffolds has attracted much attention in biological fields. In this research, some novel composites of Cu(II) or Zn(II) metal–organic framework (M-MOF) and polycaprolactone (PCL), M-MOF@PCL, have been fabricated as multifunctional scaffolds for application in the tissue engineering (TE) field. The porous three-dimensional sponges were prepared by the salt leaching method. Then, the M-MOF@PCL composite sponges have been prepared by in situ synthesis of M-MOF in the presence of the as-obtained PCL sponge to gain a new compound with proper features for biological applications. Finally, curcumin was attached to the M-MOF@PCL as a bioactive compound that can act as a wound-healing agent, anti-oxidant, and anti-inflammatory. The presence of the M-MOF in final composites was investigated by different methods such as FTIR (Fourier-transform infrared), XRD (X-ray diffraction), SEM (scanning electron microscope), EDS (energy-dispersive X-ray spectroscopy), and TEM (transmission electron microscope). SEM images confirmed the porous structure of the as-obtained composites. According to the EDS and TEM images, M-MOFs were uniformly incorporated throughout the PCL sponges. The water sorption capacities of the blank PCL, Cu-MOF@PCL, and Zn-MOF@PCL were determined as 56%, 155%, and 119%, respectively. In vivo investigation on a third-degree burn model in adult male Wistar rats exhibited an accelerated wound healing for Cu-MOF@PCL compared to with Zn-MOF@PCL and the control group.








Similar content being viewed by others
Data Availability
Not applicable.
References
Luo, H., Cha, R., Li, J., Hao, W., Zhang, Y., & Zhou, F. (2019). Advances in tissue engineering of nanocellulose-based scaffolds: A review. Carbohydrate Polymers, 224, 115144. https://doi.org/10.1016/j.carbpol.2019.115144
Annabi, N., Rana, D., Sani, E. S., Portillo-Lara, R., Gifford, J. L., Fares, M. M., et al. (2017). Engineering a sprayable and elastic hydrogel adhesive with antimicrobial properties for wound healing. Biomaterials, 139, 229–243. https://doi.org/10.1016/j.biomaterials.2017.05.011
Sharma, K., Porat, Z. E., & Gedanken, A. (2021). Designing natural polymer-based capsules and spheres for biomedical applications—a review. Polymers, 13(24), 4307. https://doi.org/10.3390/polym13244307
Simionescu, B. C., & Ivanov, D. (2016). Natural and synthetic polymers for designing composite materials. In Handbook of bioceramics and biocomposites (pp. 233–286). Springer. https://doi.org/10.1007/978-3-319-12460-5_11
Anwunobi, A. P., & Emeje, M. O. (2011). Recent applications of natural polymers in nanodrug delivery. Journal of Nanomedicine & Nanotechnology, 4(002). https://doi.org/10.4172/2157-7439.S4-002
Fadaie, M., Mirzaei, E., Geramizadeh, B., & Asvar, Z. (2018). Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydrate Polymers, 199, 628–640. https://doi.org/10.1016/j.carbpol.2018.07.061
Chandika, P., Oh, G. W., Heo, S. Y., Kim, S. C., Kim, T. H., Kim, M. S., & Jung, W. K. (2021). Electrospun porous bilayer nano-fibrous fish collagen/PCL bio-composite scaffolds with covalently cross-linked chitooligosaccharides for full-thickness wound-healing applications. Materials Science and Engineering: C, 121, 111871. https://doi.org/10.1016/j.msec.2021.111871
Mohamed, R. M., & Yusoh, K. (2016). A review on the recent research of polycaprolactone (PCL). Advanced Materials Research, 1134, 249–255. https://doi.org/10.4028/www.scientific.net/AMR.1134.249
Jiao, Z., Luo, B., Xiang, S., Ma, H., Yu, Y., & Yang, W. (2019). 3D printing of HA/PCL composite tissue engineering scaffolds. Advanced Industrial and Engineering Polymer Research, 2(4), 196–202. https://doi.org/10.1016/j.aiepr.2019.09.003
Fox, K., Ratwatte, R., Booth, M. A., Tran, H. M., & Tran, P. A. (2020). High nanodiamond content-PCL composite for tissue engineering scaffolds. Nanomaterials, 10(5), 948. https://doi.org/10.3390/nano10050948
Prahastuti, S., Hidayat, M., Hasianna, S. T., Widowati, W., Amalia, A., Yusepany, D. T., & Kusuma, H. S. W. (2019). Antioxidant potential ethanolic extract of Glycine max (l.) Merr. Var. Detam and daidzein. In Journal of Physics: Conference Series (Vol. 1374, No. 1, p. 012020). IOP Publishing. https://doi.org/10.1088/1742-6596/1374/1/012020
Sani, I. S., Rezaei, M., Khoshfetrat, A. B., & Razzaghi, D. (2021). Preparation and characterization of polycaprolactone/chitosan-g-polycaprolactone/hydroxyapatite electrospun nanocomposite scaffolds for bone tissue engineering. International Journal of Biological Macromolecules, 182, 1638–1649. https://doi.org/10.1016/j.ijbiomac.2021.05.163
Chen, L., Zhang, X., Cheng, X., Xie, Z., Kuang, Q., & Zheng, L. (2020). The function of metal–organic frameworks in the application of MOF-based composites. Nanoscale Advances, 2(7), 2628–2647. https://doi.org/10.1039/D0NA00184H
Safaei, M., Foroughi, M. M., Ebrahimpoor, N., Jahani, S., Omidi, A., & Khatami, M. (2019). A review on metal-organic frameworks: Synthesis and applications. TrAC Trends in Analytical Chemistry, 118, 401–425. https://doi.org/10.1016/j.trac.2019.06.007
Liu, B., Jiang, M., Zhu, D., Zhang, J., & Wei, G. (2022). Metal-organic frameworks functionalized with nucleic acids and amino acids for structure-and function-specific applications: A tutorial review. Chemical Engineering Journal, 428, 131118. https://doi.org/10.1016/j.cej.2021.131118
Safdar Ali, R., Meng, H., & Li, Z. (2021). Zinc-based metal-organic frameworks in drug delivery, cell imaging, and sensing. Molecules, 27(1), 100. https://doi.org/10.3390/molecules27010100
Keskin, S., & Kızılel, S. (2011). Biomedical applications of metal organic frameworks. Industrial & Engineering Chemistry Research, 50(4), 1799–1812. https://doi.org/10.1021/ie101312k
Singh, N., Qutub, S., & Khashab, N. M. (2021). Biocompatibility and biodegradability of metal organic frameworks for biomedical applications. Journal of Materials Chemistry B. https://doi.org/10.1039/D1TB01044A
Nakhaei, M., Akhbari, K., Kalati, M., & Phuruangrat, A. (2021). Antibacterial activity of three zinc-terephthalate MOFs and its relation to their structural features. Inorganica Chimica Acta, 522, 120353. https://doi.org/10.1016/j.ica.2021.120353
Li, Y., Liu, X., Tan, L., Cui, Z., Yang, X., Zheng, Y., et al. (2018). Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Advanced Functional Materials, 28(30), 1800299. https://doi.org/10.1002/adfm.201800299
Ramezani, M. R., Ansari-Asl, Z., Hoveizi, E., & Kiasat, A. R. (2020). Fabrication and characterization of Fe (III) metal-organic frameworks incorporating polycaprolactone nanofibers: potential scaffolds for tissue engineering. Fibers and Polymers, 21(5), 1013–1022. https://doi.org/10.1007/s12221-020-9523-6
Zheng, Q., Li, J., Yuan, W., Liu, X., Tan, L., Zheng, Y., et al. (2019). Metal–organic frameworks incorporated polycaprolactone film for enhanced corrosion resistance and biocompatibility of Mg Alloy. ACS Sustainable Chemistry & Engineering, 7(21), 18114–18124. https://doi.org/10.1021/acssuschemeng.9b05196
Liu, M., Wang, L., Zheng, X., & Xie, Z. (2017). Zirconium-based nanoscale metal–organic framework/poly (ε-caprolactone) mixed-matrix membranes as effective antimicrobials. ACS Applied Materials & Interfaces, 9(47), 41512–41520. https://doi.org/10.1021/acsami.7b15826
Maiti, P., & Dunbar, G. L. (2018). Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. International Journal of Molecular Sciences, 19(6), 1637. https://doi.org/10.3390/ijms19061637
Urošević, M., Nikolić, L., Gajić, I., Nikolić, V., Dinić, A., & Miljković, V. (2022). Curcumin: Biological activities and modern pharmaceutical forms. Antibiotics, 11(2), 135. https://doi.org/10.3390/antibiotics11020135
Lestari, W. W., Arvinawati, M., Martien, R., & Kusumaningsih, T. (2018). Green and facile synthesis of MOF and nano MOF containing zinc (II) and benzen 1, 3, 5-tri carboxylate and its study in ibuprofen slow-release. Materials Chemistry and Physics, 204, 141–146. https://doi.org/10.1016/j.matchemphys.2017.10.034
Emam, H. E., Darwesh, O. M., & Abdelhameed, R. M. (2018). In-growth metal organic framework/synthetic hybrids as antimicrobial fabrics and its toxicity. Colloids and Surfaces B: Biointerfaces, 165, 219–228. https://doi.org/10.1016/j.colsurfb.2018.02.028
Gandara-Loe, J., Souza, B. E., Missyul, A., Giraldo, G., Tan, J. C., & Silvestre-Albero, J. (2020). MOF-based polymeric nanocomposite films as potential materials for drug delivery devices in ocular therapeutics. ACS Applied Materials & Interfaces, 12(27), 30189–30197. https://doi.org/10.1021/acsami.0c07517
Sanjeevi, S., Shanmugam, V., Kumar, S., Ganesan, V., Sas, G., Johnson, D. J., et al. (2021). Effects of water absorption on the mechanical properties of hybrid natural fibre/phenol formaldehyde composites. Scientific Reports, 11(1), 1–11. https://doi.org/10.1038/s41598-021-92457-9
Haghshenas, M., Hoveizi, E., Mohammadi, T., & Kazemi Nezhad, S. R. (2019). Use of embryonic fibroblasts associated with graphene quantum dots for burn wound healing in Wistar rats. In Vitro Cellular & Developmental Biology-Animal, 55(4), 312–322. https://doi.org/10.1007/s11626-019-00331-w
Ren, G., He, Y., Liu, C., Ni, F., Luo, X., Shi, J., et al. (2022). Encapsulation of curcumin in ZEIN-HTCC complexes: Physicochemical characterization, in vitro sustained release behavior and encapsulation mechanism. LWT, 155, 112909. https://doi.org/10.1016/j.lwt.2021.112909
Balu, R., Kumar, T. S., Ramalingam, M., & Ramakrishna, S. (2011). Electrospun Polycaprolactone/Poly (1, 4-butylene adipate-co-polycaprolactam) blends: Potential biodegradable scaffold for bone tissue regeneration. Journal of Biomaterials and Tissue Engineering, 1(1), 30–39. https://doi.org/10.1166/jbt.2011.1004
Kemala, T., Budianto, E., & Soegiyono, B. (2012). Preparation and characterization of microspheres based on blend of poly (lactic acid) and poly (ɛ-caprolactone) with poly (vinyl alcohol) as emulsifier. Arabian Journal of Chemistry, 5(1), 103–108. https://doi.org/10.1016/j.arabjc.2010.08.003
Hosseini, M. S., Zeinali, S., & Sheikhi, M. H. (2016). Fabrication of capacitive sensor based on Cu-BTC (MOF-199) nanoporous film for detection of ethanol and methanol vapors. Sensors and Actuators B: Chemical, 230, 9–16. https://doi.org/10.1016/j.snb.2016.02.008
Loera-Serna, S., Oliver-Tolentino, M. A., de Lourdes López-Núñez, M., Santana-Cruz, A., Guzmán-Vargas, A., Cabrera-Sierra, R., et al. (2012). Electrochemical behavior of [Cu3 (BTC) 2] metal–organic framework: the effect of the method of synthesis. Journal of Alloys and Compounds, 540, 113–120. https://doi.org/10.1016/j.jallcom.2012.06.030
Homayoonnia, S., & Zeinali, S. (2016). Design and fabrication of capacitive nanosensor based on MOF nanoparticles as sensing layer for VOCs detection. Sensors and Actuators B: Chemical, 237, 776–786. https://doi.org/10.1016/j.snb.2016.06.152
Wang, X., Ma, X., Wang, H., Huang, P., Du, X., & Lu, X. (2017). A zinc (II) benzenetricarboxylate metal organic framework with unusual adsorption properties, and its application to the preconcentration of pesticides. Microchimica Acta, 184(10), 3681–3687. https://doi.org/10.1007/s00604-017-2382-1
Sindhu, K., Rajaram, A., Sreeram, K. J., & Rajaram, R. (2014). Curcumin conjugated gold nanoparticle synthesis and its biocompatibility. RSC Advances, 4(4), 1808–1818. https://doi.org/10.1039/C3RA45345F
Singh, P. K., Wani, K., Kaul-Ghanekar, R., Prabhune, A., & Ogale, S. (2014). From micron to nano-curcumin by sophorolipid co-processing: highly enhanced bioavailability, fluorescence, and anti-cancer efficacy. RSC Advances, 4(104), 60334–60341. https://doi.org/10.1039/C4RA07300B
Kundu, S., & Nithiyanantham, U. (2013). In situ formation of curcumin stabilized shape-selective Ag nanostructures in aqueous solution and their pronounced SERS activity. RSC Advances, 3(47), 25278–25290. https://doi.org/10.1039/C3RA44471F
Gotthardt, M. A., Schoch, R., Wolf, S., Bauer, M., & Kleist, W. (2015). Synthesis and characterization of bimetallic metal–organic framework Cu–Ru-BTC with HKUST-1 structure. Dalton Transactions, 44(5), 2052–2056. https://doi.org/10.1039/C4DT02491E
Fadaie, M., & Mirzaei, E. (2018). Nanofibrillated chitosan/polycaprolactone bionanocomposite scaffold with improved tensile strength and cellular behavior. Nanomedicine Journal, 5(2), 77–89. https://doi.org/10.22038/nmj.2018.005.004
Hsu, K. H., Fang, S. P., Lin, C. L., Liao, Y. S., Yoon, Y. K., & Chauhan, A. (2016). Hybrid electrospun polycaprolactone mats consisting of nanofibers and microbeads for extended release of dexamethasone. Pharmaceutical Research, 33(6), 1509–1516. https://doi.org/10.1007/s11095-016-1894-4
Ma, D., Peh, S. B., Han, G., & Chen, S. B. (2017). Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal–organic framework (MOF) UiO-66: toward enhancement of water flux and salt rejection. ACS Applied Materials & Interfaces, 9(8), 7523–7534. https://doi.org/10.1021/acsami.6b14223
Heydari, P., Zargar Kharazi, A., Asgary, S., & Parham, S. (2022). Comparing the wound healing effect of a controlled release wound dressing containing curcumin/ciprofloxacin and simvastatin/ciprofloxacin in a rat model: A preclinical study. Journal of Biomedical Materials Research Part A, 110(2), 341–352. https://doi.org/10.1002/jbm.a.37292
Menon, V. P., & Sudheer, A. R. (2007). Antioxidant and anti-inflammatory properties of curcumin. In The molecular targets and therapeutic uses of curcumin in health and disease, pp. 105–125. https://doi.org/10.1007/978-0-387-46401-5_3
Marzo, T., & La Mendola, D. (2021). The effects on angiogenesis of relevant inorganic chemotherapeutics. Current Topics In Medicinal Chemistry, 21(1), 73–86. https://doi.org/10.2174/1568026620666201126163436
Naletova, I., Greco, V., Sciuto, S., Attanasio, F., & Rizzarelli, E. (2021). Ionophore ability of carnosine and its trehalose conjugate assists copper signal in triggering brain-derived neurotrophic factor and vascular endothelial growth factor activation in vitro. International Journal of Molecular Sciences, 22(24), 13504. https://doi.org/10.3390/ijms222413504
Cangul, I. T., Gul, N. Y., Topal, A., & Yilmaz, R. (2006). Evaluation of the effects of topical tripeptide-copper complex and zinc oxide on open-wound healing in rabbits. Veterinary Dermatology, 17(6), 417–423. https://doi.org/10.1111/j.1365-3164.2006.00551.x
Coger, V., Million, N., Rehbock, C., Sures, B., Nachev, M., Barcikowski, S., et al. (2019). Tissue concentrations of zinc, iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biological Trace Element Research, 191(1), 167–176. https://doi.org/10.1007/s12011-018-1600-y
Schwartz, J. R., Marsh, R. G., & Draelos, Z. D. (2005). Zinc and skin health: overview of physiology and pharmacology. Dermatologic Surgery, 31, 837–847. https://doi.org/10.1111/j.1524-4725.2005.31729
Funding
The authors received financial support (Grant No.: SCU.SC.98.20911) from the Shahid Chamran University of Ahvaz.
Author information
Authors and Affiliations
Contributions
Zeinab Ansari-Asl: writing original—draft, conceptualization, methodology, formal analysis, investigation, resources); Soghra Nikpour: conceptualization, methodology, formal analysis, investigation, resources, visualization; Tahereh Sedaghat: conceptualization, methodology, formal analysis, investigation; Elham Hoveizi: writing original—draft, conceptualization, methodology, formal analysis.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari-Asl, Z., Nikpour, S., Sedaghat, T. et al. Preparation, Characterization, and Wound Healing Assessment of Curcumin-Loaded M-MOF (M = Cu, Zn)@Polycaprolactone Nanocomposite Sponges. Appl Biochem Biotechnol 195, 4308–4320 (2023). https://doi.org/10.1007/s12010-023-04316-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04316-0