Abstract
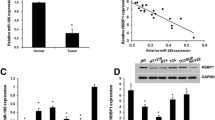
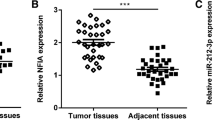
MicroRNAs (miRNAs) are critical in progression of bladder cancer (BCa). miRNA-93-5p is increased in cancers and is positively correlated with an unfavorable prognosis. But its effects on BCa remain rarely understood. This investigation aimed to dig out miRNA-93-5p affecting biological behaviors of BCa. In this research, mRNA and protein expression in cancer cells were assessed via quantitative real-time polymerase chain reaction (qRT-PCR) and western blot. Cell Counting Kit-8 (CCK-8), colony formation, scratch healing, and transwell assays were utilized to analyze cancer cell viability, colony-forming, migration, and invasion, respectively. Bioinformatics analysis predicted upstream regulatory genes and downstream target genes of miRNA-93-5p, with the targeting relationship being verified through a dual-luciferase assay. The BCa xenograft model in nude mice further investigated the effect of miRNA-93-5p and AND2-AS1 on tumor size and quality, and validated the relationship between HAND2-AS1/miRNA-93-5p/DCUN1D3. Our results displayed that miRNA-93-5p was increased in BCa cell lines. Knockdown miRNA-93-5p constrained BCa cell malignant phenotypes. HAND2-AS1 targeted miRNA-93-5p, thus restraining malignant progression of BCa cells. DCUN1D3 was found downstream of miRNA-93-5p. miRNA-93-5p modulated proliferation, migration, and invasion of BCa cells by targeting DCUN1D3. In vivo experiments disclosed that forced expression of lncRNA HAND2-AS1, and inhibited miRNA-93-5p regressed tumor growth. Meanwhile, the same as the results of cell experiments, the expression of miRNA-93-5p was downregulated, and DCUN1D3 expression was advanced in tumor tissues. To conclude, lncRNA HAND2-AS1 exerted anti-tumor effects and regulated BCa cell proliferation, invasion, and migration by targeting miRNA-93-5p/DCUN1D3.







Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding authors on reasonable request.
Materials Availability
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding authors on reasonable request.
Change history
13 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12010-023-04478-x
References
Braga, E. A., Fridman, M. V., Filippova, E. A., et al. (2021). LncRNAs in the regulation of genes and signaling pathways through miRNA-mediated and other mechanisms in clear cell renal cell carcinoma. International Journal of Molecular Sciences, 22(20),11193.
Tay, Y., Rinn, J., & Pandolfi, P. P. (2014). The multilayered complexity of ceRNA crosstalk and competition. Nature, 505(7483), 344–352.
He, R., Wang, L., Li, J., et al. (2020). Integrated analysis of a competing endogenous RNA network reveals a prognostic signature in kidney renal papillary cell carcinoma. Frontiers in Cell and Development Biology, 8, 612924.
Huang, M., Long, Y., Jin, Y., et al. (2021). Comprehensive analysis of the lncRNA-miRNA-mRNA regulatory network for bladder cancer. Translational Andrology and Urology, 10(3), 1286–1301.
Xiong, Y., Zu, X., Wang, L., et al. (2021). The VIM-AS1/miR-655/ZEB1 axis modulates bladder cancer cell metastasis by regulating epithelial-mesenchymal transition. Cancer Cell International, 21(1), 233.
Jiang, H., Bu, Q., Zeng, M., et al. (2019). MicroRNA-93 promotes bladder cancer proliferation and invasion by targeting PEDF. Urologic Oncology, 37(2), 150–157.
Zhang, K., Cai, Y., Zhou, Q., et al. (2020). Long non-coding RNA SNHG14 impedes viability, migration and invasion of endometrial carcinoma cells through modulating miR-93-5p/ZBTB7A axis. Cancer Management and Research, 12, 9515–9525.
Shyamasundar, S., Lim, J. P., & Bay, B. H. (2016). miR-93 inhibits the invasive potential of triple-negative breast cancer cells in vitro via protein kinase WNK1. International Journal of Oncology, 49(6), 2629–2636.
Yang, W., Bai, J., Liu, D., et al. (2018). MiR-93-5p up-regulation is involved in non-small cell lung cancer cells proliferation and migration and poor prognosis. Gene, 647, 13–20.
Shi, X., Liu, T. T., Yu, X. N., et al. (2020). microRNA-93-5p promotes hepatocellular carcinoma progression via a microRNA-93-5p/MAP3K2/c-Jun positive feedback circuit. Oncogene, 39(35), 5768–5781.
Shen, E., Wang, X., Liu, X., et al. (2020). MicroRNA-93-5p promotes epithelial-mesenchymal transition in gastric cancer by repressing tumor suppressor AHNAK expression. Cancer Cell International, 20, 76.
Armstrong, D. A., Green, B. B., Seigne, J. D., et al. (2015). MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Molecular Cancer, 14, 194.
Juracek, J., Peltanova, B., Dolezel, J., et al. (2018). Genome-wide identification of urinary cell-free microRNAs for non-invasive detection of bladder cancer. Journal of Cellular and Molecular Medicine, 22(3), 2033–2038.
Salmena, L., Poliseno, L., Tay, Y., et al. (2011). A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell, 146(3), 353–358.
Wieczorek, E., Reszka, E. (2018). mRNA, microRNA and lncRNA as novel bladder tumor markers. Clinica Chimica Acta; International Journal of Clinical Chemistry, 477, 141–53.
Dong, G., Wang, X., Jia, Y., et al. (2020). HAND2-AS1 works as a ceRNA of miR-3118 to suppress proliferation and migration in breast cancer by upregulating PHLPP2. BioMed Research International, 2020, 8124570.
Xu, Z., Lv, H., Wang, Y., et al. (2020). HAND2-AS1 inhibits gastric adenocarcinoma cells proliferation and aerobic glycolysis via miRNAs sponge. Cancer Management and Research, 12, 3053–3068.
Jing, G. Y., Zheng, X. Z., & Ji, X. X. (2021). lncRNA HAND2-AS1 overexpression inhibits cancer cell proliferation in hepatocellular carcinoma by downregulating RUNX2 expression. Journal of Clinical Laboratory Analysis, 35(4), e23717.
Shan, L., Liu, W., & Zhan, Y. (2021). LncRNA HAND2-AS1 exerts anti-oncogenic effects on bladder cancer via restoration of RARB as a sponge of microRNA-146. Cancer Cell International, 21(1), 361.
Chen, D., Cheng, L., Cao, H., et al. (2021). Role of microRNA-381 in bladder cancer growth and metastasis with the involvement of BMI1 and the Rho/ROCK axis. BMC Urology, 21(1), 5.
Zhang, C., Wang, W., Lin, J., et al. (2019). lncRNA CCAT1 promotes bladder cancer cell proliferation, migration and invasion. International Brazilian Journal of Urology, 45(3), 549–559.
Luo, H., Xu, C., Le, W., et al. (2019). lncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA-150. Journal of Cellular Biochemistry, 120(8), 13487–13493.
Shan, G., Zhou, X., Gu, J., et al. (2021). Downregulated exosomal microRNA-148b-3p in cancer associated fibroblasts enhance chemosensitivity of bladder cancer cells by downregulating the Wnt/beta-catenin pathway and upregulating PTEN. Cellular Oncology (Dordrecht), 44(1), 45–59.
Li, Y., Zheng, F., Xiao, X., et al. (2017). CircHIPK3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Reports, 18(9), 1646–1659.
Bi, J., Liu, H., Cai, Z., et al. (2018). Circ-BPTF promotes bladder cancer progression and recurrence through the miR-31-5p/RAB27A axis. Aging (Albany NY), 10(8), 1964–1976.
Dong, W., Bi, J., Liu, H., et al. (2019). Circular RNA ACVR2A suppresses bladder cancer cells proliferation and metastasis through miR-626/EYA4 axis. Molecular Cancer, 18(1), 95.
Gong, J., Fan, H., Deng, J., et al. (2020). LncRNA HAND2-AS1 represses cervical cancer progression by interaction with transcription factor E2F4 at the promoter of C16orf74. Journal of Cellular and Molecular Medicine, 24(11), 6015–6027.
Li, Y., Duan, Q., Gan, L., et al. (2021) microRNA-27b inhibits cell proliferation and invasion in bladder cancer by targeting engrailed-2. Bioscience Reports, 41(1),BSR20201000.
Lenis, A. T., Lec, P. M., Chamie, K., et al. (2020). Bladder cancer: A review. JAMA, 324(19), 1980–1991.
Saad, A., Hanbury, D. C., McNicholas, T. A., et al. (2001). The early detection and diagnosis of bladder cancer: A critical review of the options. European Urology, 39(6), 619–633.
Braicu, C., Cojocneanu-Petric, R., Chira, S., et al. (2015). Clinical and pathological implications of miRNA in bladder cancer. International Journal of Nanomedicine, 10, 791–800.
Yoshino, H., Seki, N., Itesako, T., et al. (2013). Aberrant expression of microRNAs in bladder cancer. Nature Reviews Urology, 10(7), 396–404.
Wang, Z., Sun, W., Li, R., et al. (2022). miRNA-93-5p in exosomes derived from M2 macrophages improves lipopolysaccharide-induced podocyte apoptosis by targeting Toll-like receptor 4. Bioengineered, 13(3), 7683–7696.
Sun, X. Y., Han, X. M., Zhao, X. L., et al. (2019). MiR-93-5p promotes cervical cancer progression by targeting THBS2/MMPS signal pathway. European Review for Medical and Pharmacological Sciences, 23(12), 5113–5121.
Li, T., Xu, Q., Wei, Y., et al. (2022). Overexpression of miRNA-93-5p promotes proliferation and migration of bladder urothelial carcinoma via inhibition of KLF9. Computational and Mathematical Methods in Medicine, 2022, 8911343.
Yang, X., Wei, X., Yi, C., et al. (2022). Long noncoding RNA HAND2-AS1 suppresses cell proliferation, migration, and invasion of bladder cancer via miR-17-5p/KLF9 Axis. DNA and Cell Biology, 41(2), 179–189.
Bi, H. Q., Li, Z. H., & Zhang, H. (2020). Long noncoding RNA HAND2-AS1 reduced the viability of hepatocellular carcinoma via targeting microRNA-300/SOCS5 axis. Hepatobiliary & Pancreatic Diseases International, 19(6), 567–574.
Ma, T., Shi, T., Huang, J., et al. (2008). DCUN1D3, a novel UVC-responsive gene that is involved in cell cycle progression and cell growth. Cancer Science, 99, 2128–2135.
Ma, T., Shi, T., Huang, J., et al. (2008). DCUN1D3, a novel UVC-responsive gene that is involved in cell cycle progression and cell growth. Cancer Science, 99(11), 2128–2135.
Acknowledgements
Not applicable.
Funding
The research was supported by high-level hospital foster grants from Fujian Provincial Hospital, Fujian Province, China (2020HSJJ13); Startup Fund for scientific research, Fujian Medical University (2019QH1175).
Author information
Authors and Affiliations
Contributions
XW and QJ contributed to conception and design of the study. TL contributed to acquisition of data. YB contributed to data analysis. RZ drafted the manuscript. RC contributed to investigation. LN contributed to visualization. LF contributed to supervision and funding acquisition. ZH revised the article and gave final approval of the version to be published.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of Fujian Provincial Hospital (K2020-12–032). All participants signed written informed consent for research purposes and publication. Ethics Committee of Zhejiang Eyoung Pharmaceutical Research and Development Center Laboratory Animal Ethics Committee approved all animal surgeries in this study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Xu, Q., Li, T. et al. Bladder Cancer Progression Is Suppressed Through the Heart and Neural Crest Derivatives Expressed 2-Antisense RNA 1/microRNA-93-5p/Defective in Cullin Neddylation 1 Domain Containing 3 Axis. Appl Biochem Biotechnol 195, 4116–4133 (2023). https://doi.org/10.1007/s12010-022-04295-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04295-8