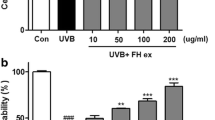
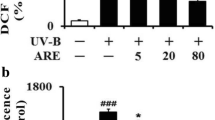
Abstract
The biological activities of Houttuynia cordata (H. cordata) fermented with Aureobasidium pullulans (A. pullulans) was investigated for human skin keratinocyte-induced chemical and photo oxidations. In this research, H2O2/UVA-induced HaCaT cell lines were treated with H. cordata water/ethanol extracts (HCW/HCE) and fermented with A. pullulans water/ethanol extracts (HCFW/HCFE). A. pullulans fermented with H. cordata (HCFW) increased in 5.4-folds of total polyphenol (HCFW 46.89 mg GAE/extract g), and 2.3-folds in flavonoids (HCFW 53.80 mg GAE/extract g) compared with water extracts of H. cordata (HCW). Further, no significant cytotoxicity for HaCaT cells showed by all the extracts of H. cordata fermented with A. pullulans. HCFW extracts have significantly lowered inflammation factors such as COX-2 and Hsp70 proteins in oxidative stressed HaCaT cells induced by H2O2 and UVA treatments. All H. cordata extracts significantly downregulated gene expression involved in oxidative stress and inflammation factors, including IL-1β, IL-6, COX-2, TNF-α, NF-κB, and MMP-1 in the H2O2/UVA-treated HaCaT cells. However, keratin-1 gene expression in the UVA-treated HaCaT cells was increased in twofolds by HCFW extracts. Further, A. pullulans fermented H. cordata extracts (HCFW/HCFE) reduced the genes involved in oxidative stresses more effectively than those of H. cordata extract only. Overall, the polyphenol-rich extracts of H. cordata fermented with A. pullulans showed synergistic protective effects for human epidermal keratinocytes to prevent photoaging and intrinsic aging by anti-oxidation and anti-inflammatory functions.
Graphical Abstract







Similar content being viewed by others
Data Availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Juzeniene, A., & Moan, J. (2012). Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinol, 4, 109–117.
Apalla, Z., Lallas, A., Sotiriou, E., Lazaridou, E., & Ioannides, D. (2017). Epidemiological trends in skin cancer. Dermatology Practical & Conceptual, 7, 1–6.
Kumar, J. P., Alam, S., Jain, A. K., Ansari, K. M., & Mandal, B. B. (2018). Protective activity of silk sericin against UV radiation-induced skin damage by downregulating oxidative stress. ACS Applied Bio Materials, 1, 2120–2132.
Vile, G. F., & Tyrrell, R. M. (1995). UVA radiation-induced oxidative damage to lipids and proteins in vitro and in human skin fibroblasts is dependent on iron and singlet oxygen. Free Radical Biology & Medicine, 18, 721–730.
Ding, Y., Jiratchayamaethasakul, C., & Lee, S.-H. (2020). Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. International Journal of Molecular Sciences, 21, 4619.
Orioli, D., & Dellambra, E. (2018). Epigenetic regulation of skin cells in natural aging and premature aging diseases. Cells, 7, 268.
Jeon, S., & Choi, M. (2018). Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomedical Dermatology, 2, 1–8.
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., & Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling, 20, 1126–1167.
Liu, T., Zhang, L., Joo, D., & Sun, S.-C. (2017). NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy, 2, 17023.
Won, J.-H., Im, H.-T., Kim, Y.-H., Yun, K.-J., Park, H.-J., Choi, J.-W., & Lee, K.-T. (2006). Anti-inflammatory effect of buddlejasaponin IV through the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via the NF-kappaB inactivation. British Journal of Pharmacology, 148, 216–225.
Keum, Y. S., Kim, H. G., Bode, A. M., Surh, Y. J., & Dong, Z. (2013). UVB-induced COX-2 expression requires histone H3 phosphorylation at Ser10 and Ser28. Oncogene, 32, 444–452.
Fischer, S. M., Pavone, A., Mikulec, C., Langenbach, R., & Rundhaug, J. E. (2007). Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Molecular Carcinogenesis, 46, 363–371.
Brennan, M., Bhatti, H., Nerusu, K. C., Bhagavathula, N., Kang, S., Fisher, G. J., Varani, J., & Voorhees, J. J. (2003). Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin¶. Photochemistry and photobiology, 78, 43–48.
Ha, J. H., Kim, A. R., Lee, K.-S., Xuan, S. H., Kang, H. C., Lee, D. H., Cha, M. Y., Kim, H. J., An, M., & Park, S. N. (2019). Anti-Aging activity of Lavandula angustifolia extract fermented with pediococcus pentosaceus DK1 isolated from Diospyros kaki fruit in UVB-irradiated human skin fibroblasts and analysis of principal components. Journal of microbiology and biotechnology, 29, 21–29.
Kwon, K.-R., Alam, M. B., Park, J.-H., Kim, T.-H., & Lee, S.-H. (2019). Attenuation of UVB-induced photo-aging by polyphenolic-rich Spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients, 11, 1341.
Rahman, M., Alam, K., Zaki Ahmad, M., Gupta, G., Afzal, M., Akhter, S., Kazmi, I., Jalees Ahmad, F., & Anwar, F. (2012). Classical to current approach for treatment of psoriasis: A review. Endocrine Metabolic & Immune Disorders-Drug Targets Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders, 12, 287–302.
Diniyah, N., Alam, M. B., Choi, H.-J., & Lee, S.-H. (2020). Lablab purpureus protects HaCaT Cells from oxidative stress-induced cell death through Nrf2-mediated heme oxygenase-1 expression via the activation of p38 and ERK1/2. International Journal of Molecular Sciences, 21, 8583.
Kim, K. H., Park, S. J., Lee, Y. J., Lee, J. E., Song, C. H., Choi, S. H., Ku, S. K., & Kang, S. J. (2015). Inhibition of UVB-induced skin damage by exopolymers from Aureobasidium pullulans SM-2001 in Hairless Mice. Basic & Clinical Pharmacology & Toxicology, 116, 73–86.
Hayashi, K., Kamiya, M., & Hayashi, T. (1995). Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta medica, 61, 237–241.
Miyata, M., Koyama, T., & Yazawa, K. (2010). Water extract of Houttuynia cordata Thunb. leaves exerts anti-obesity effects by inhibiting fatty acid and glycerol absorption. Journal of nutritional science and vitaminology, 56, 150–156.
Kim, G. S., Kim, D. H., Lim, J. J., Lee, J. J., Han, D. Y., Lee, W. M., Jung, W. C., Min, W. G., Won, C. G., & Rhee, M. H. (2008). Biological and antibacterial activities of the natural herb Houttuynia cordata water extract against the intracellular bacterial pathogen salmonella within the RAW 264.7 macrophage. Biological and Pharmaceutical Bulletin, 31, 2012–2017.
Lu, H. M., Liang, Y. Z., Yi, L. Z., & Wu, X. J. (2006). Anti-inflammatory effect of Houttuynia cordata injection. Journal of Ethnopharmacology, 104, 245–249.
Kim, J. M., Hwang, I.-H., Jang, I.-S., Kim, M., Bang, I. S., Park, S. J., Chung, Y.-J., Joo, J.-C., & Lee, M.-G. (2017). Houttuynia cordata Thunb promotes activation of HIF-1A–FOXO3 and MEF2A pathways to induce apoptosis in human HepG2 hepatocellular carcinoma cells. Integrative Cancer Therapies, 16, 360–372.
Ahn, J., Chae, H.-S., Chin, Y.-W., & Kim, J. (2017). Alkaloids from aerial parts of Houttuynia cordata and their anti-inflammatory activity. Bioorganic & Medicinal Chemistry Letters, 27, 2807–2811.
Sekita, Y., Murakami, K., Yumoto, H., Hirao, K., Amoh, T., Fujiwara, N., Hirota, K., Fujii, H., Matsuo, T., & Miyake, Y. (2017). Antibiofilm and anti-inflammatory activities of houttuynia cordata decoction for oral care. Evidence-Based Complementary and Alternative Medicine, 2017, 2850947.
Chun, J. M., Nho, K. J., Kim, H. S., Lee, A. Y., Moon, B. C., & Kim, H. K. (2014). An ethyl acetate fraction derived from Houttuynia cordata extract inhibits the production of inflammatory markers by suppressing NF-кB and MAPK activation in lipopolysaccharide-stimulated RAW 264.7 macrophages. BMC Complementary and Alternative Medicine, 14, 234.
Lee, H. J., Seo, H. S., Kim, G. J., Jeon, C. Y., Park, J. H., Jang, B. H., Park, S. J., Shin, Y. C., & Ko, S. G. (2013). Houttuynia cordata Thunb inhibits the production of pro-inflammatory cytokines through inhibition of the NFκB signaling pathway in HMC-1 human mast cells. Molecular Medicine reports, 8, 731–736.
Zhu, H., Lu, X., Ling, L., Li, H., Ou, Y., Shi, X., Lu, Y., Zhang, Y., & Chen, D. (2018). Houttuynia cordata polysaccharides ameliorate pneumonia severity and intestinal injury in mice with influenza virus infection. Journal of ethnopharmacology, 218, 90–99.
Bozoudi, D., & Tsaltas, D. (2018). The multiple and versatile roles of Aureobasidium pullulans in the vitivinicultural sector. Fermentation, 4, 85.
Muramatsu, D., Iwai, A., Aoki, S., Uchiyama, H., Kawata, K., Nakayama, Y., Nikawa, Y., Kusano, K., Okabe, M., & Miyazaki, T. (2012). β-glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS ONE, 7, e41399.
Kwon, R. H., & Ha, B. J. (2012). Increased flavonoid compounds from fermented Houttuynia cordata using isolated six of Bacillus from traditionally fermented Houttuynia cordata. Toxicological Research, 28, 117–122.
Lee, S.-G., Kang, S., Lee, K.-Y., Park, K.-L. & Kang, H. (2017). Antioxidants and anti-inflammatory effects of fermented Houttuynia cordata Thunb and scoria mixture extract. Biomedical Science Letters, 23, 355–361.
Lee, S. J., Hu, W., Lee, E. J., Choi, J. Y., & Koo, O. K. (2018). Polyphenolic Profile of fermented Houttuynia cordata Thunb. and Overall contribution to antioxidant and lipolytic activities. Food Engineering Progress, 22, 295–303.
Hayashi, N., Shoubayashi, Y., Kondo, N., & Fukudome, K. (2019). Hydrothermal processing of β-glucan from Aureobasidium pullulans produces a low molecular weight reagent that regulates inflammatory responses induced by TLR ligands. Biochemical and Biophysical Research Communications, 511, 318–322.
Kawata, K., Iwai, A., Muramatsu, D., Aoki, S., Uchiyama, H., Okabe, M., Hayakawa, S., Takaoka, A., & Miyazaki, T. (2015). Stimulation of macrophages with the β-glucan produced by Aureobasidium pullulans promotes the secretion of tumor necrosis factor-related apoptosis inducing ligand (TRAIL). PLoS ONE, 10, e0124809.
Khaleghnezhad, V., Yousefi, A. R., Tavakoli, A., & Farajmand, B. (2019). Interactive effects of abscisic acid and temperature on rosmarinic acid, total phenolic compounds, anthocyanin, carotenoid and flavonoid content of dragonhead (Dracocephalum moldavica L.). Scientia Horticulturae, 250, 302–309.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free radical biology and medicine, 26, 1231–1237.
Singh, M., Lee, K. E., Vinayagam, R., & Kang, S. G. (2021). Antioxidant and antibacterial profiling of pomegranate-pericarp extract functionalized-zinc oxide nanocomposite. Biotechnology and Bioprocess Engineering, 26, 728–737.
Kwon, N., Lee, K. E., Singh, M., & Kang, S. G. (2021). Suitable primers for GAPDH reference gene amplification in quantitative RT-PCR analysis of human gene expression. Gene Reports, 24, 101272.
Napagoda, M. T., Malkanthi, B. M. A. S., Abayawardana, S. A. K., Qader, M. M., & Jayasinghe, L. (2016). Photoprotective potential in some medicinal plants used to treat skin diseases in Sri Lanka. BMC Complementary and Alternative Medicine, 16, 479.
Ahmady, A., Amini, M. H., Zhakfar, A. M., Babak, G., & Sediqi, M. N. (2020). Sun protective potential and physical stability of herbal sunscreen developed from Afghan medicinal plants. Turkish Journal of Pharmaceutical Sciences, 17, 285–292.
Jakovljević, V. D., & Preservation. (2021). An overview of antioxidant capacity, total phenolics, and amino acids of Aureobasidium pullulans GM6. Journal of Food Processing and Preservation, 45, e15227.
Palanisamy, U., Cheng, H. M., Masilamani, T., Subramaniam, T., Ling, L. T., & Radhakrishnan, A. K. (2008). Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chemistry, 109, 54–63.
Thitilertdecha, N., Teerawutgulrag, A., & Rakariyatham, N. (2008). Antioxidant and antibacterial activities of Nephelium lappaceum L. extracts. LWT-Food Science and Technology, 41, 2029–2035.
Chou, S.-C., Su, C.-R., Ku, Y.-C., & Wu, T.-S. (2009). The constituents and their bioactivities of Houttuynia cordata. Chemical and Pharmaceutical Bulletin, 57, 1227–1230.
Senawong, T., Khaopha, S., Misuna, S., Komaikul, J., Senawong, G., Wongphakham, P., & Yunchalard, S. (2014). Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. ScienceAsia, 40, 420–7.
Ahn, J., Chae, H.-S., Chin, Y.-W., Kim, J. J. B. & Letters, M. C. (2017). Alkaloids from aerial parts of Houttuynia cordata and their anti-inflammatory activity. Bioorganic & Medicinal Chemistry Letters, 27, 2807–2811.
Chaiprasongsuk, A., & Panich, U. (2022). Role of phytochemicals in skin photoprotection via regulation of Nrf2. Frontiers in Pharmacology, 13, 823881.
Shin, D., Lee, Y., Huang, Y.-H., Lim, H.-W., Jang, K., Kim, D.-D., Lim, C.-J. (2018). Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complementary and Alternative Medicine, 18, 1–10.
Wu, Z., Deng, X., Hu, Q., Xiao, X., Jiang, J., Ma, X., & Wu, M. (2021). Houttuynia cordata Thunb: An ethnopharmacological review. Frontiers in Pharmacology, 12, 714694.
Sánchez-Moreno, C. (2002). Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food science and technology international, 8, 121–137.
Liu, J., Zhu, X., Yang, D., Li, R., & Jiang, J. (2021). Effect of heat treatment on the anticancer activity of Houttuynia cordata Thunb aerial stem extract in human gastric cancer SGC-7901 cells. Nutrition and Cancer, 73, 160–168.
Senawong, T., Khaopha, S., Misuna, S., Komaikul, J., Senawong, G., Wongphakham, P., & Yunchalard, S. (2014). Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. ScienceAsia, 40, 420–427.
De Jager, T. L., Cockrell, A. E., & Du Plessis, S. S. (2017). in Ultraviolet light in human health (pp. 15–23). Springer.
Zhang, J., Wang, W., & Mao, X. (2020). Chitopentaose protects HaCaT cells against H2O2-induced oxidative damage through modulating MAPKs and Nrf2/ARE signaling pathways. Journal of Functional Foods, 72, 104086.
Fanjul-Fernández, M., Folgueras, A. R., Cabrera, S., & López-Otín, C. (2010). Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1803, 3–19.
Pittayapruek, P., Meephansan, J., Prapapan, O., Komine, M., & Ohtsuki, M. (2016). Role of matrix metalloproteinases in photoaging and photocarcinogenesis. International Journal of Molecular Sciences, 17, 868.
Matsuda, M., Hoshino, T., Yamashita, Y., Tanaka, K.-I., Maji, D., Sato, K., Adachi, H., Sobue, G., Ihn, H., & Funasaka, Y. (2010). Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. Journal of Biological Chemistry, 285, 5848–5858.
Simon, M. M., Reikerstorfer, A., Schwarz, A., Krone, C., Luger, T. A., Jäättelä, M., & Schwarz, T. (1995). Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. The Journal of clinical investigation, 95, 926–933.
Fuller, B. (2019). Role of PGE-2 and other inflammatory mediators in skin aging and their inhibition by topical natural anti-inflammatories. Cosmetics, 6, 6.
Balupillai, A., Kanimozhi, G., Khan, H. A., Alhomida, A. S. & Prasad, N. R. (2020). Opuntiol prevents photoaging of mouse skin via blocking inflammatory responses and collagen degradation. Oxidative Medicine and Cellular Longevity, 2020, 5275178.
Tang, S.-C., Liao, P.-Y., Hung, S.-J., Ge, J.-S., Chen, S.-M., Lai, J.-C., Hsiao, Y.-P., & Yang, J.-H. (2017). Topical application of glycolic acid suppresses the UVB induced IL-6, IL-8, MCP-1 and COX-2 inflammation by modulating NF-κB signaling pathway in keratinocytes and mice skin. Journal of dermatological science, 86, 238–248.
Maes, M. (2008). The cytokine hypothesis of depression: Inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuroendocrinology Letters, 29, 287–291.
Wlaschek, M., Heinen, G., Poswig, A., Schwarz, A., Krieg, T., & Scharffetter-Kochanek, K. (1994). UVA-induced autocrine stimulation of fibroblast-derived collagenase/mmp-1 by interrelated loops ofinterleukin–1 andinterleukin–6. Photochemistry and Photobiology, 59, 550–556.
Oh, J. H., Karadeniz, F., Lee, J. I., Kim, H. R., Seo, Y., & Kong, C.-S. (2020). Antiphotoaging effect of (2′ S)-Columbianetin from Corydalis heterocarpa in UVA-irradiated human dermal fibroblasts. Applied Sciences, 10, 2568.
Zhao, J., Cheung, P. C. (2011). Fermentation of β-glucans derived from different sources by bifidobacteria: Evaluation of their bifidogenic effect. Journal of agricultural and food chemistry, 59, 5986–5992.
Acknowledgements
The authors thank the Core Research Support Center for Natural Products and Medical Materials at Yeungnam University, Gyeongsan, the Republic of Korea, for technical support regarding PCR analysis using the RT-PCR and Freeze Dryer (FDA5518). This work has been supported by Stemforce Inc., Gyeongsan, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Nakyoung Kwon: experiment. Ramachandran Vinayagam: writing-original draft, data curation, and editing. Geum Sook Do: data curation, writing, and editing of the manuscript. Kyung Eun Lee: experiment design, perform experiments, methodology development, data curation, and material support. Sang Gu Kang: experiment design, writing and editing the manuscript, and project supervision. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable. This article does not contain any with human participants or animals.
Consent to Participate
Not applicable because this study does not involve human participants.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwon, N., Vinayagam, R., Do, G.S. et al. Protective Effects of Fermented Houttuynia cordata Against UVA and H2O2-Induced Oxidative Stress in Human Skin Keratinocytes. Appl Biochem Biotechnol 195, 3027–3046 (2023). https://doi.org/10.1007/s12010-022-04241-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04241-8