Abstract
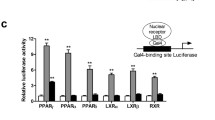
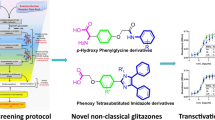
The clinically used glitazones (rosiglitazone and pioglitazone) for type 2 diabetes mellitus therapy have been linked to serious side effects such as fluid retention, congestive heart failure, weight gain, bone loss, and an increased risk of bladder cancer. The complete activation of PPAR-γ receptors in target tissues is linked to these effects. Many studies have demonstrated that partial PPAR-γ activators (GW0072, PAT5A, GQ16) give equivalent therapeutic benefits to full PPAR-γ agonists without the associated side effects. These breakthroughs cleared the path for the development of partial agonists or selective PPAR-γ modulators (SPPARγMs). This study combined pharmacophore modeling, molecular docking, and an adipogenesis experiment to identify thiazolidine analogs as SPPARMs/partial agonists. A custom library of 220 molecules was created and virtual screened to discover 90 compounds as SPPARγMs/ partial agonists. The chosen eight compounds were synthesized and tested for adipogenesis using 3T3L1 cell lines. These compounds’ partial agonistic activity was evaluated in 3T3L1 cell lines by comparing their capacity to stimulate PPAR-γ mediated adipogenesis to that of a full agonist, rosiglitazone. The findings of the adipogenesis experiment demonstrate that all eight compounds examined had a partial potential to stimulate adipogenesis when compared to the full agonist, rosiglitazone. The current investigation identified eight possible PPAR-γ partial agonists or SPPARγMs that may be effective in the treatment of type 2 diabetes mellitus.
















Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism, and elimination
- AF-2:
-
Activation factor-2
- AUAC:
-
Area under the accumulation curve
- BEDROC:
-
Boltzmann-enhanced discrimination of ROC
- DEX:
-
Dexamethasone
- DIAD:
-
Diisopropyl azodicarboxylate
- DMEM:
-
Dulbecco’s modified Eagle medium
- DMSO:
-
Dimethyl sulfoxide
- EF1%:
-
Enrichment factor 1%
- FBS:
-
Fetal bovine serum
- IBMX:
-
3-Isobutyl-1-methylxanthine
- LBD:
-
Ligand binding domain
- PPARs:
-
Peroxisome proliferator-activated receptors
- PPh3:
-
Triphenylphosphine
- ROC:
-
Receiver operating characteristic
- SDS:
-
Sodium dodecyl sulfate
- SPPARγMs:
-
Selective PPAR-γ modulators
- T2DM:
-
Type 2 diabetes mellitus
- THF:
-
Tetrahydrofuran
- TZDs:
-
Thiazolidinediones
- VS:
-
Virtual screening
- XP:
-
Extra precision
References
Tenenbaum, A., Fisman, E. Z., & Motro, M. (2003). Metabolic syndrome and type 2 diabetes mellitus: Focus on peroxisome proliferator activated receptors (PPAR). Cardiovascular Diabetology, 2, 4.
Wang, S., Lin, Y., Gao, L., Yang, Z., Lin, J., Ren, S., Li, F., Chen, J., Wang, Z., Dong, Z., Sun, P., & Wu, B. (2022). PPAR-gamma integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics, 12, 1589–1606.
Tan, Y., Muise, E. S., Dai, H., Raubertas, R., Wong, K. K., Thompson, G. M., Wood, H. B., Meinke, P. T., Lum, P. Y., & Thompson, J. R. (2012). Novel transcriptome profiling analyses demonstrate that selective peroxisome proliferator-activated receptor γ (PPARγ) modulators display attenuated and selective gene regulatory activity in comparison with PPARγ full agonists. Molecular Pharmacology, 82, 68–79.
Tontonoz, P., Hu, E., & Spiegelman, B. M. (1994). Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell, 79, 1147–1156.
Knouff, C., & Auwerx, J. (2004). Peroxisome proliferator-activated receptor-γ calls for activation in moderation: Lessons from genetics and pharmacology. Endocrine Reviews, 25, 899–918.
Helal, M., Ali, M. A., Nadrin, A. H., Awad, Y. I., Younis, N. K., Alasyed, B. M., Jamal, M., Eid, D. H., Soliman, H. A., Eissa, S. A. and Ragab, M. (2022). Association between IRS-1, PPAR-gamma and LEP genes polymorphisms and growth traits in rabbits. Anim Biotechnol, 1–9.
Lange, N. F., Graf, V., Caussy, C. & Dufour, J. F. (2022). PPAR-targeted therapies in the treatment of non-alcoholic fatty liver disease in diabetic patients. International Journal Molecular Science, 23.
Teixeira, C., Sousa, A. P., Santos, I., Rocha, A. C., Alencastre, I., Pereira, A. C., Martins-Mendes, D., Barata, P., Baylina, P. & Fernandes, R. (2022). Enhanced 3T3-L1 differentiation into adipocytes by pioglitazone pharmacological activation of peroxisome proliferator activated receptor-gamma (PPAR-gamma). Biology (Basel), 11.
Faghfouri, A. H., Khajebishak, Y., Payahoo, L., Faghfuri, E., & Alivand, M. (2021). PPAR-gamma agonists: Potential modulators of autophagy in obesity. European Journal of Pharmacology, 912, 174562.
Liu, C., Xiong, Q., Li, Q., Lin, W., Jiang, S., Zhang, D., Wang, Y., Duan, X., Gong, P., & Kang, N. (2022). CHD7 regulates bone-fat balance by suppressing PPAR-gamma signaling. Nature Communications, 13, 1989.
Soccio, R. E., Chen, E. R., & Lazar, M. A. (2014). Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell metabolism, 20, 573–591.
Yokoi, T. (2010), in Adverse drug reactions: Handbook of Experimental Pharmacology, 419–435.
Kroker, A. J., & Bruning, J. B. (2015). Review of the structural and dynamic mechanisms of PPARγpartial agonism. PPAR Research, 2015, 1–15.
Jaeschke, H. (2007). Troglitazone hepatotoxicity: Are we getting closer to understanding idiosyncratic liver injury? Toxicological Sciences, 97, 1–3.
Cheng, D., Gao, H. & Li, W. (2018). Wpływ długotrwałego stosowania rozyglitazonu na zdarzenia sercowo-naczyniowe — przegląd systematyczny i metaanaliza. Endokrynologia Polska, 69.
Ryder, R. E. J. (2015). Pioglitazone has a dubious bladder cancer risk but an undoubted cardiovascular benefit. Diabetic Medicine, 32, 305–313.
Korhonen, P., Heintjes, E. M., Williams, R., Hoti, F., Christopher, S., Majak, M., Kool-Houweling, L., Strongman, H., Linder, M., Dolin, P. and Bahmanyar, S. (2016) Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. Bmj.
Ferwana, M., Firwana, B., Hasan, R., Al-Mallah, M. H., Kim, S., Montori, V. M., & Murad, M. H. (2013). Pioglitazone and risk of bladder cancer: A meta-analysis of controlled studies. Diabetic Medicine, 30, 1026–1032.
Allen, T., Zhang, F., Moodie, S. A., Clemens, L. E., Smith, A., Gregoire, F., Bell, A., Muscat, G. E., & Gustafson, T. A. (2006). Halofenate is a selective peroxisome proliferator–activated receptor γ modulator with antidiabetic activity. Diabetes, 55, 2523–2533.
Nofziger, C., & Blazer-Yost, B. L. (2009). PPARγ agonists, modulation of ion transporters, and fluid retention. Journal of the American Society of Nephrology, 20, 2481–2483.
Elasy, T. A., & Griffin, M. (2004). Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association: Response to Nesto. Diabetes Care, 27, 2096–2096.
Cariou, B., Charbonnel, B., & Staels, B. (2012). Thiazolidinediones and PPARγ agonists: Time for a reassessment. Trends in Endocrinology & Metabolism, 23, 205–215.
Zoete, V., Grosdidier, A., & Michielin, O. (2007). Peroxisome proliferator-activated receptor structures: Ligand specificity, molecular switch and interactions with regulators. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1771, 915–925.
Wright, M. B., Bortolini, M., Tadayyon, M., & Bopst, M. (2014). Minireview: Challenges and opportunities in development of PPAR agonists. Molecular Endocrinology, 28, 1756–1768.
Step, S. E., Lim, H.-W., Marinis, J. M., Prokesch, A., Steger, D. J., You, S.-H., Won, K.-J., & Lazar, M. A. (2014). Anti-diabetic rosiglitazone remodels the adipocyte transcriptome by redistributing transcription to PPARγ-driven enhancers. Genes & Development, 28, 1018–1028.
Trujillo, M. E., & Scherer, P. E. (2006). Adipose tissue-derived factors: Impact on health and disease. Endocrine Reviews, 27, 762–778.
Choi, J. H., Banks, A. S., Estall, J. L., Kajimura, S., Boström, P., Laznik, D., Ruas, J. L., Chalmers, M. J., Kamenecka, T. M., & Blüher, M. (2010). Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature, 466, 451.
Sharma, A. M., & Staels, B. (2006). Peroxisome proliferator-activated receptor γ and adipose tissue—Understanding obesity-related changes in regulation of lipid and glucose metabolism. The Journal of Clinical Endocrinology & Metabolism, 92, 386–395.
Marciano, D. P., Kuruvilla, D. S., Boregowda, S. V., Asteian, A., Hughes, T. S., Garcia-Ordonez, R., Corzo, C. A., Khan, T. M., Novick, S. J., & Park, H. (2015). Pharmacological repression of PPARγ promotes osteogenesis. Nature Communications, 6, 7443.
Rizos, C., Elisaf, M., Mikhailidis, D., & Liberopoulos, E. (2009). How safe is the use of thiazolidinediones in clinical practice? Expert Opinion on Drug Safety, 8, 15–32.
Nolte, R. T., Wisely, G. B., Westin, S., Cobb, J. E., Lambert, M. H., Kurokawa, R., Rosenfeld, M. G., Willson, T. M., Glass, C. K., & Milburn, M. V. (1998). Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ. Nature, 395, 137.
Thangavel, N., Al Bratty, M., Akhtar Javed, S., Ahsan, W. and Alhazmi, H. A. (2017). Targeting peroxisome proliferator-activated receptors using thiazolidinediones: Strategy for design of novel antidiabetic drugs. International journal of medicinal chemistry, 2017.
Bruning, J. B., Chalmers, M. J., Prasad, S., Busby, S. A., Kamenecka, T. M., He, Y., Nettles, K. W., & Griffin, P. R. (2007). Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure, 15, 1258–1271.
Capelli, D., Cerchia, C., Montanari, R., Loiodice, F., Tortorella, P., Laghezza, A., Cervoni, L., Pochetti, G. & Lavecchia, A. (2016). Structural basis for PPAR partial or full activation revealed by a novel ligand binding mode. Scientific Reports, 6.
Sheu, S.-H., Kaya, T., Waxman, D. J., & Vajda, S. (2005). Exploring the binding site structure of the PPARγ ligand-binding domain by computational solvent mapping†. Biochemistry, 44, 1193–1209.
Farce, A., Renault, N., & Chavatte, P. (2009). Structural insight into PPARγ ligands binding. Current Medicinal Chemistry, 16, 1768–1789.
Montanari, R., Saccoccia, F., Scotti, E., Crestani, M., Godio, C., Gilardi, F., Loiodice, F., Fracchiolla, G., Laghezza, A., Tortorella, P., Lavecchia, A., Novellino, E., Mazza, F., Aschi, M., & Pochetti, G. (2008). Crystal structure of the peroxisome proliferator-activated receptor γ (pparγ) ligand binding domain complexed with a novel partial agonist: A new region of the hydrophobic pocket could be exploited for drug design. Journal of Medicinal Chemistry, 51, 7768–7776.
Thaggikuppe Krishnamurthy, P., JogheeNanjanChandrasekar, M., & JogheeNanjan, M. (2013). Newer approaches to the discovery of glitazones. Mini-Reviews in Organic Chemistry, 10, 66–72.
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A., & Brown, M. (2000). Cofactor dynamics and sufficiency in estrogen receptor–regulated transcription. Cell, 103, 843–852.
Smith, C. L., & O’malley, B. W. (2004). Coregulator function: A key to understanding tissue specificity of selective receptor modulators. Endocrine Reviews, 25, 45–71.
Krishnamurthy Praveen, T., JogheeNanjanChandrasekar, M., & JogheeNanjan, M. (2013). Novel glitazones with diverse peroxisome proliferator activated receptor modulatory potential. Current Bioactive Compounds, 9, 221–234.
Denizot, F., & Lang, R. (1986). Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunological Methods, 89, 271–277.
Zebisch, K., Voigt, V., Wabitsch, M., & Brandsch, M. (2012). Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Analytical Biochemistry, 425, 88–90.
Berger, J. P., Petro, A. E., Macnaul, K. L., Kelly, L. J., Zhang, B. B., Richards, K., Elbrecht, A., Johnson, B. A., Zhou, G., & Doebber, T. W. (2003). Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator. Molecular Endocrinology, 17, 662–676.
Yi, W., Shi, J., Zhao, G., Zhou, X. E., Suino-Powell, K., Melcher, K., & Xu, H. E. (2017). Identification of a novel selective pparγ ligand with a unique binding mode and improved therapeutic profile in vitro. Scientific Reports, 7, 41487.
Chen, Y., Ma, H., Zhu, D., Zhao, G., Wang, L., Fu, X. & Chen, W. (2017). Discovery of novel insulin sensitizers: Promising approaches and targets. PPAR Research, 2017.
Pinaire, J. A., Miller, A. R., & Gregoire, F. M. (2008). Development of synthetic modulators of PPARs: Current challenges and future opportunities. PPAR Research, 2008, 7.
Kubota, N., Terauchi, Y., Miki, H., Tamemoto, H., Yamauchi, T., Komeda, K., Satoh, S., Nakano, R., Ishii, C., & Sugiyama, T. (1999). PPARγ mediates high-fat diet–induced adipocyte hypertrophy and insulin resistance. Molecular cell, 4, 597–609.
Chaudhary, S., Dube, A., Kothari, V., Sachan, N., & Upasani, C. D. (2012). NS-1: A novel partial peroxisome proliferator-activated receptor γ agonist to improve insulin sensitivity and metabolic profile. European Journal of Pharmacology, 684, 154–160.
Oberfield, J. L., Collins, J. L., Holmes, C. P., Goreham, D. M., Cooper, J. P., Cobb, J. E., Lenhard, J. M., Hull-Ryde, E. A., Mohr, C. P., & Blanchard, S. G. (1999). A peroxisome proliferator-activated receptor γ ligand inhibits adipocyte differentiation. Proceedings of the National Academy of Sciences, 96, 6102–6106.
Sime, M., Allan, A. C., Chapman, P., Fieldhouse, C., Giblin, G. M., Healy, M. P., Lambert, M. H., Leesnitzer, L. M., Lewis, A., & Merrihew, R. V. (2011). Discovery of GSK1997132B a novel centrally penetrant benzimidazole PPARγ partial agonist. Bioorganic & Medicinal Chemistry Letters, 21, 5568–5572.
Ebdrup, S., Pettersson, I., Rasmussen, H. B., Deussen, H.-J., Frost Jensen, A., Mortensen, S. B., Fleckner, J., Pridal, L., Nygaard, L., & Sauerberg, P. (2003). Synthesis and biological and structural characterization of the dual-acting peroxisome proliferator-activated receptor α/γ agonist ragaglitazar. Journal of Medicinal Chemistry, 46, 1306–1317.
Nagy, L., & Schwabe, J. W. (2004). Mechanism of the nuclear receptor molecular switch. Trends in Biochemical Sciences, 29, 317–324.
Liu, C., Feng, T., Zhu, N., Liu, P., Han, X., Chen, M., Wang, X., Li, N., Li, Y., & Xu, Y. (2015). Identification of a novel selective agonist of PPARγ with no promotion of adipogenesis and less inhibition of osteoblastogenesis. Scientific Reports, 5, 9530.
Burgermeister, E., Schnoebelen, A., Flament, A., Benz, Jr., Stihle, M., Gsell, B., Rufer, A., Ruf, A., Kuhn, B., & Märki, H. P. (2006). A novel partial agonist of peroxisome proliferator-activated receptor-γ (PPARγ) recruits PPARγ-coactivator-1α, prevents triglyceride accumulation, and potentiates insulin signaling in vitro. Molecular Endocrinology, 20, 809–830.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., KS, N., Byran, G. et al. Identification of Selective PPAR-γ Modulators by Combining Pharmacophore Modeling, Molecular Docking, and Adipogenesis Assay. Appl Biochem Biotechnol 195, 1014–1041 (2023). https://doi.org/10.1007/s12010-022-04190-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04190-2