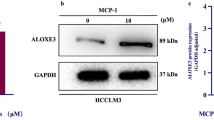
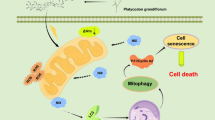
Abstract
Understanding the principle of regulated cell death (RCD) such as ferroptosis and apoptosis provides opportunities to overcome sorafenib resistance of HCC. Complexin II (CPLX2) is involved in calcium-dependent fusion of vesicles and plasma membrane, and recent studies showed CPLX2 is involved in cancer progression. However, the expression and function of CPLX2 are unclear in hepatocellular carcinoma (HCC). qPCR and western blotting assays were used to detect the levels of CPLX2. MTT and colony formation assays were used to detect cell viability. The contents of iron, ROS, MDA, and GSH were used to evaluate the function of CPLX2 on ferroptosis, while the flow cytometry and TUNEL assays were used to evaluate the role of CPLX2 on apoptosis. Our analysis showed CPLX2 is significantly upregulated in HCC, which predicts poor overall survival (OS), progression-free survival (PFS), relapse-free survival (RFS), and disease-specific survival (DSS) for patients with HCC. Further function enrichment analysis of genes related to CPLX2 showed CPLX2 is involved in the NRF2 pathway. Downregulation of CPLX2 can inhibit NRF2 expression and the transcription of its downstream genes, which confirms that CPLX2 is involved in NRF2 pathway. Cell viability assay showed that ferroptosis and apoptosis inhibitors can reverse the inhibition effect of CPLX2-knockdown on cell survival, respectively. And downregulation of CPLX2 significantly promotes the contents of iron, ROS, and MDA, while inhibiting the GSH level of HCC cell lysate, suggesting CPLX2 involved in ferroptosis. Moreover, downregulation of CPLX2 promotes the apoptosis of HCC cells by flow cytometry and TUNEL assay. And upregulation of NRF2 can partly reverse the inhibitory effect of CPLX2-downregulation on ferroptosis and apoptosis. Finally, we found downregulation of CPLX2 aggravates cell death induced by sorafenib. CPXL2 regulates ferroptosis and apoptosis through NRF2 pathway, and CPLX2 knockdown promotes cell death induced by sorafenib. CPLX2 might be an effective target for therapy patients with HCC.







Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Liang, C., Zhang, X., Yang, M., & Dong, X. (2019). Recent progress in ferroptosis inducers for cancer therapy. Advanced Materials, 31(51), e1904197. https://doi.org/10.1002/adma.201904197
Tan, M. L., Ooi, J. P., Ismail, N., Moad, A. I., & Muhammad, T. S. (2009). Programmed cell death pathways and current antitumor targets. Pharmaceutical Research, 26(7), 1547–1560. https://doi.org/10.1007/s11095-009-9895-1
Bialik, S., Zalckvar, E., Ber, Y., Rubinstein, A. D., & Kimchi, A. (2010). Systems biology analysis of programmed cell death. Trends in Biochemical Sciences, 35(10), 556–564. https://doi.org/10.1016/j.tibs.2010.04.008
Ouyang, L., Shi, Z., Zhao, S., Wang, F. T., Zhou, T. T., Liu, B., et al. (2012). Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Proliferation, 45(6), 487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Angeli, J. P. F., Shah, R., Pratt, D. A., & Conrad, M. (2017). Ferroptosis inhibition: Mechanisms and opportunities. Trends in Pharmacological Sciences, 38(5), 489–498. https://doi.org/10.1016/j.tips.2017.02.005
Vanden Berghe, T., Linkermann, A., Jouan-Lanhouet, S., Walczak, H., & Vandenabeele, P. (2014). Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nature Reviews Molecular Cell Biology, 15(2), 135–147. https://doi.org/10.1038/nrm3737
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Li, J., Cao, F., Yin, H. L., Huang, Z. J., Lin, Z. T., Mao, N., et al. (2020). Ferroptosis: Past, present and future. Cell Death & Disease, 11(2), 88. https://doi.org/10.1038/s41419-020-2298-2
Koppula, P., Zhuang, L., & Gan, B. (2020). Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein & Cell. https://doi.org/10.1007/s13238-020-00789-5
Kang, R., Kroemer, G., & Tang, D. (2019). The tumor suppressor protein p53 and the ferroptosis network. Free Radical Biology & Medicine, 133, 162–168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
Xie, Y., Hou, W., Song, X., Yu, Y., Huang, J., Sun, X., et al. (2016). Ferroptosis: Process and function. Cell Death and Differentiation, 23(3), 369–379. https://doi.org/10.1038/cdd.2015.158
Savaskan, N. E., Ufer, C., Kuhn, H., & Borchert, A. (2007). Molecular biology of glutathione peroxidase 4: From genomic structure to developmental expression and neural function. Biological Chemistry, 388(10), 1007–1017. https://doi.org/10.1515/BC.2007.126
Xu, T., Ding, W., Ji, X., Ao, X., Liu, Y., Yu, W., et al. (2019). Molecular mechanisms of ferroptosis and its role in cancer therapy. Journal of Cellular and Molecular Medicine, 23(8), 4900–4912. https://doi.org/10.1111/jcmm.14511
Dodson, M., Castro-Portuguez, R., & Zhang, D. D. (2019). NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biology, 23, 101107. https://doi.org/10.1016/j.redox.2019.101107
Rojo de la Vega, M., Chapman, E., & Zhang, D. D. (2018). NRF2 and the hallmarks of cancer. Cancer Cell., 34(1), 21–43. https://doi.org/10.1016/j.ccell.2018.03.022
Niture, S. K., & Jaiswal, A. K. (2012). Nrf2 protein up-regulates antiapoptotic protein Bcl-2 and prevents cellular apoptosis. Journal of Biological Chemistry, 287(13), 9873–9886. https://doi.org/10.1074/jbc.M111.312694
Niture, S. K., & Jaiswal, A. K. (2013). Nrf2-induced antiapoptotic Bcl-xL protein enhances cell survival and drug resistance. Free Radical Biology & Medicine, 57, 119–131. https://doi.org/10.1016/j.freeradbiomed.2012.12.014
Tsuru, E., Oryu, K., Sawada, K., Nishihara, M., & Tsuda, M. (2019). Complexin 2 regulates secretion of immunoglobulin in antibody-secreting cells. Immun Inflamm Dis., 7(4), 318–325. https://doi.org/10.1002/iid3.276
Komatsu, H., Kakehashi, A., Nishiyama, N., Izumi, N., Mizuguchi, S., Yamano, S., et al. (2013). Complexin-2 (CPLX2) as a potential prognostic biomarker in human lung high grade neuroendocrine tumors. Cancer Biomarkers, 13(3), 171–180. https://doi.org/10.3233/CBM-130336
Makuuchi, R., Terashima, M., Kusuhara, M., Nakajima, T., Serizawa, M., Hatakeyama, K., et al. (2017). Comprehensive analysis of gene mutation and expression profiles in neuroendocrine carcinomas of the stomach. Biomedical Research, 38(1), 19–27. https://doi.org/10.2220/biomedres.38.19
Bikkavilli, R. K., Zerayesus, S. A., Van Scoyk, M., Wilson, L., Wu, P. Y., Baskaran, A., et al. (2017). K-homology splicing regulatory protein (KSRP) promotes post-transcriptional destabilization of Spry4 transcripts in non-small cell lung cancer. Journal of Biological Chemistry, 292(18), 7423–7434. https://doi.org/10.1074/jbc.M116.757906
Yang, H., Cho, M. E., Li, T. W., Peng, H., Ko, K. S., Mato, J. M., et al. (2013). MicroRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. The Journal of Clinical Investigation, 123(1), 285–298. https://doi.org/10.1172/JCI63861
Latunde-Dada, G. O. (2017). Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochimica et Biophysica Acta - General Subjects, 1861(8), 1893–1900. https://doi.org/10.1016/j.bbagen.2017.05.019
Song, Y., Miao, Y., & Song, C. P. (2014). Behind the scenes: The roles of reactive oxygen species in guard cells. New Phytologist, 201(4), 1121–1140. https://doi.org/10.1111/nph.12565
Jiang, L., Hickman, J. H., Wang, S. J., & Gu, W. (2015). Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle, 14(18), 2881–2885. https://doi.org/10.1080/15384101.2015.1068479
Yang, W. S., & Stockwell, B. R. (2008). Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chemistry & Biology, 15(3), 234–245. https://doi.org/10.1016/j.chembiol.2008.02.010
Hadian, K., & Stockwell, B. R. (2020). SnapShot: Ferroptosis. Cell, 181(5), 1188-e1. https://doi.org/10.1016/j.cell.2020.04.039
Tang, W., Chen, Z., Zhang, W., Cheng, Y., Zhang, B., Wu, F., et al. (2020). The mechanisms of sorafenib resistance in hepatocellular carcinoma: Theoretical basis and therapeutic aspects. Signal Transduction and Targeted Therapy, 5(1), 87. https://doi.org/10.1038/s41392-020-0187-x
Ling, S., Song, L., Fan, N., Feng, T., Liu, L., Yang, X., et al. (2017). Combination of metformin and sorafenib suppresses proliferation and induces autophagy of hepatocellular carcinoma via targeting the mTOR pathway. International Journal of Oncology, 50(1), 297–309. https://doi.org/10.3892/ijo.2016.3799
Sun, X., Niu, X., Chen, R., He, W., Chen, D., Kang, R., et al. (2016). Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology, 64(2), 488–500. https://doi.org/10.1002/hep.28574
Nie, J., Lin, B., Zhou, M., Wu, L., & Zheng, T. (2018). Role of ferroptosis in hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology, 144(12), 2329–37. https://doi.org/10.1007/s00432-018-2740-3
Funding
The study was supported by Science and Technology Plan Project of Guangzhou (Grant No. 202002030044) and Fund of Affiliated Huadu Hospital, Southern Medical University (People’s Hospital of Huadu District) (Grant No. 2020B03).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: J Zhao, T.S. Cao; (II) administrative support: T.S. Cao; (III) provision of study materials or patients: P.Y. Xu, X.L. Zhong; (IV) collection and assembly of data: J. Zhao, H. Li; (V) data analysis and interpretation: L.J. Du, P. Fang, C.F. Zhang; (VI) manuscript writing: all authors; (VII) final approval of manuscript: all authors. H.L. designed the study and revised and edited the manuscript. J.Z. analyzed the data, interpreted the results, and wrote the manuscript. T.S.C. designed and guided the whole experiment. L.J.D., P.F., M.J.L., and C.F.Z. helped with the sample preparation and performed the experiments, and P.Y.X., X.L.Z., and L.J.T. helped with the patients’ clinical assessment and compiled the clinical details. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Our study was approved by the Ethics Committee of the People’s Hospital of Huadu District. All procedures involving people comply with the ethical standards of the institutional and/or national committee for research ethics and the 1964 Helsinki Declaration and its subsequent changes or comparable ethical standards.
Consent to Participate
This article does not contain any studies with human participants performed by any of the authors.
Consent for Publication
All other authors have read the manuscript and have agreed to submit it in its current form for consideration for publication in the Applied Biochemistry and Biotechnology.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Zhao, J., Zhong, Xl. et al. CPLX2 Regulates Ferroptosis and Apoptosis Through NRF2 Pathway in Human Hepatocellular Carcinoma Cells. Appl Biochem Biotechnol 195, 597–609 (2023). https://doi.org/10.1007/s12010-022-04135-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04135-9