Abstract
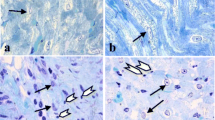
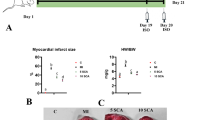
The study examined the protective effects of swertiamarin on rats with experimentally induced myocardial infarction. Three to six week-old male albino Wistar rats were used in this study and experimental myocardial infarction (MI) was induced using isoproterenol. Our results showed that swertiamarin restored the alteration in heart weight, body weight, and heart weight/tibia length ratio of MI-induced rats to basal levels significantly (p < 0.05). Swertiamarin significantly (p < 0.05) restored the levels of cardiac pathophysiological marker creatine kinase (CKMB), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine transaminase (ALT), and cardiac troponin I (cTn-1) to near normalcy in MI-induced rats. Levels of oxidative stress markers malondialdehyde (MDA), protein carbonyls (PC), and levels of Vitamin C and Vitamin E were significantly (p < 0.05) reverted to near basal levels in MI-induced rats by swertiamarin. Levels of the antioxidant glutathione (GSH) and antioxidant enzymes which include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-s-transferase (GST), glutathione reductase (GR), and plasma total antioxidant capacity (TAC) were (p < 0.05) brought to near normalcy in MI-induced rats by swertiamarin. Levels of sodium (Na), potassium (k), and calcium (Ca) ATPases were significantly (p < 0.05) restored to near normalcy in MI-induced rats by swertiamarin. Status of pro-inflammatory cytokines including tumor necrosis factor (TNF-α), interleukin-6 (IL-6), and histological aberrations were also significantly (p < 0.05) restored to near normalcy in MI-induced rats by swertiamarin. Together, our results concluded that swertiamarin exerts significant cardioprotective functions in experimental MI in rats.









Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- pH:
-
Potential of hydrogen
- M:
-
Molar
- mg:
-
Milligram
- µg:
-
Microgram
- kg:
-
Kilogram
- ISO:
-
Isoproterenol hydrochloride
- MI:
-
Myocardial infarction
- CK-MB:
-
Creatine kinase
- LDH:
-
Lactate dehydrogenase
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine transaminase
- cTn 1:
-
Cardiac troponin I
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- GST:
-
Glutathione-s-transferase
- GR:
-
Glutathione reductase
- TAC:
-
Plasma total antioxidant capacity plasma
- Na:
-
Sodium (Na)
- k:
-
Potassium
- Ca:
-
Calcium
- TNF-α:
-
Tumor necrosis factor
- IL-6:
-
Interleukin-6
- BSA:
-
Bovine serum albumin
- NaCl:
-
Sodium chloride
- ISO:
-
Isoproterenol hydrochloride
- MDA:
-
Malondialdehyde
- PC:
-
Protein carbonyls
- Vit-C:
-
Vitamin C
- Vit-E:
-
Vitamin E
References
Kate Meier,et al. (2009). Chapter 41 - Myocardial infarction. Small Animal Critical Care Medicine, 174–176.
Quan, N., Wang, L., Chen, X., et al. (2018). Sestrin2 prevents age-related intolerance to post myocardial infarction via AMPK/PGC-1α pathway. The Journal of Molecular and CellularCardiology, 115, 170–178.
Spath, N. B., Mills, N. L., & Cruden, N. L. (2016). Novel cardioprotective and regenerative therapies in acute myocardial infarction: A review of recent and ongoing clinical trials. Future Cardiology, 12(6), 655–672.
Fordyce, C. B., Gersh, B. J., Stone, G. W., & Granger, C. B. (2015). Novel therapeutics in myocardial infarction: Targeting microvascular dysfunction and reperfusion injury. Trends Pharmacological Sciences, 36(9), 605–616.
Leor, J., Tuvia, S., Guetta, V., et al. (2009). Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. Journal of the American College of Cardiology, 54, 1014–1023.
Stone, G. W., Dixon, S. R., Grines, C. L., et al. (2007). Predictors of infarct size after primary coronary angioplasty in acute myocardial infarction from pooled analysis from four contemporary trials. American Journal of Cardiology, 100(9), 1370–1375.
Satessa, G. D., Lenjisa, J. L., Gebremariam, E. T., & Woldu, M. A. (2015). Stem cell therapy for myocardial infarction: Challenges and prospects. The Journal of Stem Cell Research & Therapy 5(3), 1–5.
Ojha, S., Al Taee, H., Goyal, S., et al. (2016). Cardioprotective potentials of plant-derived small molecules against doxorubicin associated cardiotoxicity. Oxidative Medicine and Cellular Longevity, 2016, 5724973.
Otręba, M., Kośmider, L., & Rzepecka-Stojko, A. (2021). Polyphenols’ cardioprotective potential: Review of rat fibroblasts as well as rat and human cardiomyocyte cell lines research. Molecules (Basel, Switzerland), 26(4), 774.
Ray, P. D., Huang, P. W., & Tsuji, Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling, 24, 981–990.
Kim, H., Yun, J., & Kwon, S. M. (2016). Therapeutic strategies for oxidative stress-related cardiovascular diseases: Removal of excess reactive oxygen species in adult stem cells. Oxidative Medicine and Cellular Longevity, 2016, 2483163.
Moris, D., Spartalis, M., Spartalis, E., Karachaliou, G. S., Karaolanis, G. I., Tsourouflis, G., Tsilimigras, D. I., Tzatzaki, E., & Theocharis, S. (2017). The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Annals of Translational Medicine, 5(16), 326.
Swirski, F. K., & Nahrendorf, M. (2013). Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science, 339(6116), 161–166.
Vasu, V. T., Modi, H., Thaikoottathil, J. V., & Gupta, S. (2005). Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume aqueous extract in cholesterol fed rats. Journal of Ethnopharmacology, 101(1–3), 277–282.
Jaishree, V., Badami, S., Rupesh Kumar, M., & Tamizhmani, T. (2009). Antinociceptive activity of swertiamarin isolated from Enicostemma axillare. Phytomedicine, 16(2–3), 227–232.
Muhamad Fadzil, N. S., Sekar, M., Gan, S. H., Bonam, S. R., Wu, Y. S., Vaijanathappa, J., Ravi, S., Lum, P. T., & Dhadde, S. B. (2021). Chemistry, pharmacology and therapeutic potential of swertiamarin – A promising natural lead for new drug discovery and development. Drug Design, Development and Therapy., 15, 2721–2746.
Hazra, S. K., Sarkar, T., Salauddin, M., Sheikh, H. I., Pati, S., & Chakraborty, R. (2020). Characterization of phytochemicals, minerals and in vitro medicinal activities of bael (Aegle marmelos L.) pulp and differently dried edible leathers. Heliyon, 6(10), e05382. https://doi.org/10.1016/j.heliyon.2020.e05382
Sarkar, T., Salauddin, M., & Chakraborty, R. (2020). In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. Journal of agriculture and food research, 2, 100081. https://doi.org/10.1016/j.jafr.2020.100081
Sathish, V., Ebenezar, K. K., & Devaki, T. (2003). Synergistic effect of nicorandil and amlodipine on tissue defense system during experimental myocardial infarction in rats. Molecular and Cellular Biochemistry, 243, 33–138.
Schretter, C. E., Vielmetter, J., Bartos, I., Marka, Z., Marka, S., Argade, S., & Mazmanian, S. K. (2018). A gut microbial factor modulates locomotor behaviour in Drosophila. Nature, 563(7731), 402–406. https://doi.org/10.1038/s41586-018-0634-9
Aydin, S., Ugur, K., Aydin, S., Sahin, İ, & Yardim, M. (2019). Biomarkers in acute myocardial infarction: Current perspectives. Vascular Health Risk Management, 15, 1–10. https://doi.org/10.2147/VHRM.S166157
Hill, M. F., Palace, V. P., Kaur, K., Kumar, D., Khaper, N., & Singal, P. K. (2005). Reduction in oxidative stress and modulation of heart failure subsequent to myocardial infarction in rats. Experimental and clinical cardiology, 10(3), 146–153.
Mason, S. A., Trewin, A. J., Parker, L., & Wadley, G. D. (2020). Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox biology, 35, 101471. https://doi.org/10.1016/j.redox.2020.101471
Noichri, Y., Chalghoum, A., Chkioua, L., Baudin, B., Ernez, S., Ferchichi, S., & Miled, A. (2013). Low erythrocyte catalase enzyme activity is correlated with high serum total homocysteine levels in Tunisian patients with acute myocardial infarction. Diagnostic pathology, 8, 68. https://doi.org/10.1186/1746-1596-8-68
Lim, C. C., Bryan, N. S., Jain, M., Garcia-Saura, M. F., Fernandez, B. O., Sawyer, D. B., Handy, D. E., Loscalzo, J., Feelisch, M., & Liao, R. (2009). Glutathione peroxidase deficiency exacerbates ischemia-reperfusion injury in male but not female myocardium: insights into antioxidant compensatory mechanisms. American Journal of Physiology Heart and circulatory physiology, 297(6), H2144–H2153. https://doi.org/10.1152/ajpheart.00673.2009
Pahwa, S., Sharma, R., & Singh, B. (2017). Role of glutathione S-transferase in coronary artery disease patients with and without type 2 diabetes mellitus. Journal of Clinical Diagnostic Research, 11(1), BC05–BC08. https://doi.org/10.7860/JCDR/2017/23846.9281
Zuzak, E., Horecka, A., Kiełczykowska, M., Dudek, A., Musik, I., Kurzepa, J., Kurzepa, J. (2017). Glutathione level and glutathione reductase activity in serum of coronary heart disease patients. Journal of Pre-Clinical and Clinical Research, 11(2), 103–105. https://doi.org/10.26444/jpccr/81277
Zhu, F., Li, Y., Zhang, J., Piao, C., Liu, T., Li, H.-H., et al. (2013). Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One, 8(9), 74535.
Bezerra, O. C., França, C. M., Rocha, J. A., et al. (2017). Cholinergic stimulation improves oxidative stress and inflammation in experimental myocardial infarction. Scientific Reports, 7, 13687.
Gökdemir, et al. (2013). The role of oxidative stress and inflammation in the early evaluation of acute non-ST-elevation myocardial infarction: An observational study. Oxidative stress and inflammation in NSTEMI, 13, 131–136.
Nian, M., Lee, P., Khaper, N., & Liu, P. (2004). Inflammatory cytokines and postmyocardial infarction remodeling. Circulation Research, 94, 1543–1553.
Pisoschi, A. M., & Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of medicinal chemistry, 97, 55–74.
Emingway, H., Feder, G.S., Fitzpatrick, N.K., et al. (2017). Using nationwide ‘big data’ from linked electronic health records to help improve outcomes in cardiovascular diseases: 33 studies using methods from epidemiology, informatics, economics and social science in the ClinicAl disease research using LInked Bespoke studies and electronic health Records (CALIBER) programme. Southampton (UK): NIHR Journals Library; (Programme Grants for Applied Research, No. 5.4.) Chapter 11, Acute myocardial infarction: evidence of treatment benefits beyond those provided by clinical trials – Further opportunities to improve outcomes. Available from:
Sen, A., Vardaxis, I., Lindqvist, B. H., et al. (2019). Systematic assessment of prescribed medications and short-term risk of myocardial infarction – A pharmacopeia-wide association study from Norway and Sweden. Scientific Reports, 9, 8257.
Chang, X., Zhang, T., Zhang, W., Zhao, Z., & Sun, J. (2020). Natural drugs as a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxidative Medicine and Cellular Longevity, 2020(20), 5430407.
Parwani, K., Patel, F., Patel, D., & Mandal, P. (2021). Protective effects of swertiamarin against methylglyoxal-induced epithelial-mesenchymal transition by improving oxidative stress in rat kidney epithelial (NRK-52E) cells. Molecules, 26, 2748.
Wu, et al. (2017). Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cellular Physiology Biochemistry, 241, 2242–2254.
Jaisree, & Badami, S. (2010). Anti-oxidant and hepatoprotective effect of swertiamarin from Enicostemma axillary against D-galactosamine induced acute liver damage in rats. Journal of Ethnopharmacology, 130(1), 103–6.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Wu, S., Ibrahim, I.A.A. et al. Cardioprotective Role of Swertiamarin, a Plant Glycoside Against Experimentally Induced Myocardial Infarction via Antioxidant and Anti-inflammatory Functions. Appl Biochem Biotechnol 195, 5394–5408 (2023). https://doi.org/10.1007/s12010-022-04094-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04094-1