Abstract
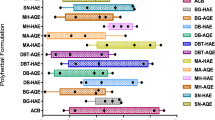
Antidiabetic polyherbal formulations (APH) are used in management of diabetes mellitus (DM). High glucose levels in DM are related to oxidative stress leading to its associated complications. Therefore, assessing antioxidant activity of various APH might unveil an antioxidant-rich formulation for management of DM and its associated complications. Subsequently selecting an antioxidant assessment method is a challenging aspect, considering various in vitro assays working with diverse mechanism of action. Therefore, present study aims to validate the sensitivity/capacity of different antioxidant assay, thereby assessing the antioxidant potential of 9-APH. Obtained results revealed the ABTS·+ values were higher compared to DPPH+ assay. I-9-HAE (DPPH+: IC50 53.31 µg/ml), NK-HAE (ABTS·+: IC50 2.71 µg/ml), and MN-HAE (FRAP and TAC) exhibited highest antioxidant capacity. A significant correlation was obtained between TPC-DPPH+ (r2: 0.8187****). Furthermore, three APH with better antiradical potential was chosen for various in vitro and in silico method, for validating scientific antidiabetic propensities. Among the tested extracts, I-9-HAE (α-amylase inhibition: IC50 831.84 µg/ml) and MN-HAE (α-glucosidase inhibition: IC50 558.64 µg/ml and antiglycation: IC50 883.74 µg/ml) have showed highest antihyperglycemic and antiglycation properties. Finally, the secondary-metabolites of selected APH were screened through literature search, Lipinski rule, ADMET, and ProTox-II. Subsequently, in molecular docking for the selected 9 secondary metabolites, highest binding affinity was observed in apigenin-7-glucuronide for DPPiv (− 9.6), GLP-1 (− 8.8), NADPH (− 8.7), and HSA (− 9.4). Thus, obtained result proposes synergistic interaction with high antioxidant potential of the selected 3-APH and can be considered an alternative for management of DM, where multiple secondary metabolites exert holistic biological effects. Furthermore, our study also provides data on sensitivity/capacity of different in vitro antioxidant assays.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Moein, S., Pimoradloo, E., Moein, M., & Vessal, M. (2017). Evaluation of antioxidant potentials and α-amylase inhibition of different fractions of Labiatae plants extracts: As a model of antidiabetic compounds properties. BioMed Research International, 2017, 1–8.
Grover, K. J., Yadav, S., & Vats, V. (2002). Medicinal plants of India with anti-diabetic potential. Journal of Ethnopharmacology, 81, 81–100.
Defronzo, A. R. (2004). Pathogenesis of type 2 diabetes mellitus. Medical Clinics of North America, 88, 787–835.
Thakur, P., Kumar, A. and Kumar, A. (2017) Targeting oxidative stress through antioxidants in Diabetes mellitus. Journal of Drug Targeting, 19.
Suriyavathana, M., Parameswari, G., & PenislusShiyan, S. (2012). Biochemical and antimicrobial study of Boerhavia erecta and Chromolaena odorata (L.) king and Robinson. International Journal of Pharmaceutical Science and Research, 3, 465–468.
Erik, J. H., Maggie, K., Diamond, S., & Elizabeth, M. M. (2011). Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radical Biology & Medicine, 51, 993–999.
Paolisso, G., D’Amore, A., Giugliano, D., Ceriello, A., Varricchio, M., & D’Onofrio, F. (1993). Pharmacologic doses of vitamin E improve insulin action in healthy subjects and non-insulin dependent diabetic patients. American Journal of Clinical Nutrition, 57, 650–656.
Paolisso, G., D’Amore, A., Balbi, V., Volpe, C., Galzerano, D., Giugliano, D., Sgambato, S., Varricchio, M., & D’Onofrio, F. (1994). Plasma vitamin C affects glucose homeostasis in healthy subjects and non-insulin dependent diabetics. American Journal of Physiology, 266, 261–268.
Bajaj, S., & Khan, A. (2012). Antioxidants and diabetes. Indian journal of endocrinology and metabolism, 16, 267–271.
Telapolu, S., Kalachavedu, M., Punnoose, M. A., & Bilikere, D. (2018). MD-1, a poly herbal formulation indicated in diabetes mellitus ameliorates glucose uptake and inhibits adipogenesis – An invitro study. BMC Complementary and Alternative Medicine, 18, 1–11.
Pisoschi, M. A., & Negulescu, P. G. (2011). Methods for total antioxidant activity determination: A review. Biochem & Anal Biochem, 1, 1–10.
Sadeer, N. B., Montesano, D., Albrizio, S., Zengin, G., & Mahomoodally, M. F. (2020). The versatility of antioxidant assays in food science and safety-chemistry, applications, strengths, and limitations. Antioxidants, 9, 1–39.
Singleton, V. L., Orthofer, R., & Lamuela, R. R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology, 299, 152–178.
Meda, N. T. R., Bangou, M. J., Bakasso, S., Millogo-Rasolodimby, J., & Nacoulma, O. G. (2013). Antioxidant activity of phenolic and flavonoid fractions of Cleome gynandra and Maerua angolensis of Burkina Faso. Journal of Applied Pharmaceutical Science., 3, 36–42.
Blois, M. S. (1958). Antioxidant determination by the use of a stable free radical. Nature, 181, 1199–1200.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine, 26, 1231–1237.
Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry, 239, 70–76.
Prieto, P., Pineda, M., & Anguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a Phosphomolybdenum Complex: Specific application to the determination of Vitamin E. Analytical Biochemistry, 269, 337–341.
Butala, A. M., Kukkupuni, S. K., & Vishnuprasad, C. N. (2017). Ayurvedic anti-diabetic formulation Lodhrasavam inhibits alpha- amylase, alpha-glucosidase and suppresses adipogenic activity in vitro. Journal of Ayurveda and Integrative Medicine, 8, 145–151.
Saleem, B., Islam, M., Saeed, H., Imtiaz, F., Asghar, M., Saleem, Z., Mehmood, A., & Naheed, S. (2018). Investigations of Acacia modesta Wall. leaves for in vitro anti-diabetic, proliferative and cytotoxic effects. Brazilian Journal of Pharmaceutical Sciences, 54, 1–8.
Clyne, A., Yang, L., Yang, M., May, B., & Yang, A. W. H. (2020). Molecular docking and network connections of active compounds from the classical herbal formula Ding Chuan Tang. PeerJ, 8, 1–25.
Oso, J. B., Adeoye, O. A., & Olaoye, F. I. (2020). Pharmacoinformatics and hypothetical studies on allicin, curcumin, and gingerol as potential candidates against COVID-19-associated proteases. Journal of Biomolecular Structure and Dynamics, 40, 389–400.
Banerjee, P., Eckert, A. O., Schrey, A. K., & Preissner, R. (2018). ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Research, 46, W257–W263.
Daina, A., Michielin, O., & Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7, 42717.
Das, S. K., Mahanta, S., Tanti, B., Tag, H., & Hui, P. K. (2022). Identification of phytocompounds from Houttuynia cordata Thunb. as potential inhibitors for SARS-CoV-2 replication proteins through GC–MS/LC–MS characterization, molecular docking and molecular dynamics simulation. Molecular Diversity, 26, 365–388.
Do, D. Q., Angkawijaya, E. A., Tran-Nguyen, L. P., Huynh, H. L., Soetaredjo, E. F., Ismadji, S., & Ju, Y. (2014). Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. Journal of food and drug analysis, 22, 296–302.
Madike, N. L., Takaidza, S., & Pillay, M. (2017). Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. International Journal of Pharmacognosy and Phytochemical Research, 9, 1300–1308.
Comert, D. E., & Gokmen, V. (2017). Antioxidants bound to an insoluble food matrix: Their analysis, regeneration behaviour, and physiological importance. Comprehensive Reviews in Food Science and Food Safety, 16, 382–399.
Fraga, C. G., Galleano, M., Verstraeten, S. V., & Oteiza, I. P. (2010). Basic biochemical mechanisms behind the health benefits of polyphenols. Molecular Aspects of Medicine, 31, 435–445.
Ojo, C. M., Osunsanmi, O. F., Zaharare, E. G., Mosa, A. R., Cele, N. D., Oboh, O. M., & Opoku, R. A. (2019). In-vitro anti-diabetic and antioxidant efficacy of methanolic extract of Encephalartos ferox leaves. Pharmacognosy Journal, 11, 455–460.
Fresco, P., Borges, F., Diniz, C., & Marques, M. P. M. (2006). New insights on the anticancer properties of dietary polyphenols. Medicinal Research Review, 26, 747–766.
Sowndhararajan, K., & Kang, C. S. (2013). Free-radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn. Saudi Journal of Biological Sciences, 20, 319–325.
Govindan, P., & Muthukrishnan, S. (2013). Evaluation of total phenolic content and free radical scavenging activity of Boerhavia erecta. Journal of Acute Medicine, 3, 103–109.
Havsteen, H. B. (2002). The biochemistry and medical significance of the flavonoids. Pharmacology and Therapeutics, 96, 67–202.
Salunke, S., Pande, V., Kendre, P., & Vibhute, S. (2015). Effect of standardized polyherbal formulations on blood glucose, body weight, food and water consumption of rat. Pharmaceutical Sciences, 21, 56–63.
Johansen, S. J., Harris. K. A., Rychly, J. D. and Ergul, A. (2005). Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovascular Diabetology, 4 (5).
Chaves, N., Santiago, A., & Alías, J.-C. (2020). Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants, 9, 1–15.
Kumar, S., & Pandey, K. A. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 2013, 16.
Majumder, P., & Paridhavi, M. (2018). A novel polyherbal formulation hastens diabetic wound healing with potent antioxidant potential: A comprehensive pharmacological investigation. Natural Products Chemistry and Research, 6, 1–9.
Franco, R. R., De Carvalho, S. D., de Moura, R. B. F., Justino, B. A., Silva, G. C. H., Peixoto, G. L., & Espindola, S. F. (2018). Evaluation of α-amylase, α-glucosidase and lipase inhibitory activities of some medicinal plants used in type-2 Diabetes Mellitus and its anti-glycation and antioxidant roles. Journal of Ethnopharmacology, 17, 140–146.
Gulati, V., Harding, H. I., & Palombo, A. E. (2012). Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycaemia. BMC Complementary and Alternative Medicine, 12, 1–9.
Bajpai, K. V., Baek, H. K., & Kang, C. S. (2017). Antioxidant and free radical scavenging activities of taxoquinone, aditerpenoid isolated from Metasequoia glyptostroboides. South African Journal of Botany, 111, 93–98.
Floegel, A., Kim, D. O., Chung, S. J., Koo, I. S., & Chun, K. O. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis, 24, 1043–1048.
Pulido, R., Bravo, L., & Saura-Calixto, F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal Agriculture Food Chemistry, 48, 3396–3402.
Halvorsen, B. L., Carlsen, M. H., Phillips, K. M., Bohn, S. K., Holte, K., Jacobs, D. R., Jr., & Blomhoff, R. (2006). Content of redox-active compounds (i.e., antioxidants) in foods consumed in the United States. American Journal of Clinical Nutrition, 84, 95–135.
Anyasor, G. N., Idowu, D. P., & Nabofa, W. (2020). Evaluation of the hepatoprotective effect of oral administration of aqueous fraction of methanolic extract of Costus afer leaves during induction of hepatocellular carcinoma with diethylnitrosamine in rats. Comparative Clinical Pathology, 29, 733–744.
Karadeniz, F., Burdurlu, H. S., Koca, N., & Soyer, Y. (2005). Antioxidant activity of selected fruits and vegetable grown in Turkey. Turkish Journal of Agriculture and Forestry, 29, 297–303.
Bhatti, M. Z., Ali, A., Ahmad, A., Saeed, A., & Malik, S. A. (2015). Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Research Notes, 8, 279.
Clarke, G., Ting, N. K., Wiart, C., & Fry, J. (2012). High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all three assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants, 2, 1–10.
Wang, M., Li, J., Rangarajan, M., Shao, Y., LaVoie, J. E., Huang, T. C., & Ho, T. C. (1998). Antioxidative phenolic compounds from sage (Salvia offinalis). Journal of Agricultural and Food Chemistry, 46, 4869–4873.
Silva, E., Souza, J., Rogez, H., Rees, J. F., & Larondelle, Y. (2007). Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chemistry, 101, 1012–1018.
Sultana, B., Anwar, F., & Przybylski, R. (2007). Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. Trees. Food Chemistry, 104, 1106–1114.
Iqbal, S., Younas, U., Sirajuddin, C. K. W., Sarfraz, R. A., & Uddin, K. (2012). Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.): A comparative study. International Journal Molecular Science, 13, 6651–6664.
Oyedemi, S. O., Oyedemi, B. O., Jeh, I. I., Ohanyerem, P. E., Coopoosamy, R. M., & Aiyegoro, O. A. (2017). Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in Southeastern Nigeria. The scientific world Journal, 2017, 1–11.
Pirbalouti, G. A., Siahpoosh, A., Setayesh, M., & Craker, L. (2014). Antioxidant activity, total phenolic and flavonoid contents of some medicinal and aromatic plants used as herbal teas and condiments in Iran. Journal of medicinal food, 17, 1151–1157.
Duraiswamy, A., Shanmugasundaram, D., Sasikumar, S. C., Cherian, S. M., & Cherian, K. M. (2016). Development of an antidiabetic formulation (ADJ6) and its inhibitory activity against a-amylase and a-glucosidase. Journal of Traditional and Complementary Medicine, 6, 204–208.
Bhatia, A., Singh, B., Arora, R., & Arora, S. (2019). In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complementary and Alternative Medicine, 19, 74.
Thrikawala, V. S., Deraniyagala, S. A., Fernando, C. D., & Udukala, D. N. (2018). In vitro α-amylase and protein glycation inhibitory activity of the aqueous extract of Flueggea leucopyrus Willd. Journal of Chemistry, 2018, 1–7.
Bernardo, J., Malheiro, I., Videira, R. A., Valentao, P., Santos, C. A., Veiga, F., & Andrade, P. B. (2021). Trichilia catigua and Turnera diffusa extracts: In vitro inhibition of tyrosinase, antiglycation activity and effects on enzymes and pathways engaged in the neuroinflammatory process. Journal of Ethnopharmacology, 271, 1–8.
Sattar, N. A., Hussain, F., Iqbal, T., & Sheikh, M. A. (2012). Determination of in vitro antidiabetic effects of Zingiber officinale Roscoe. Brazilian Journal of Pharmaceutical Science, 48, 601–607.
Koch, E. R., & Deo, P. (2016). Nutritional supplements modulate fluorescent protein-bound advanced glycation end products and digestive enzymes related to type 2 diabetes mellitus. BMC Complementary and Alternative Medicine, 16, 1–7.
Abdelli, I., Hassani, F., Brikci, B. S., & Ghalem, S. (2020). In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoides verticillata components harvested from western Algeria. Journal of Biomolecular Structure and Dynamics, 39, 3263–3276.
Almi, Z., Belaidi, S., Melkemi, N., Boughdiri, S. and Belkhir, L. (2015) Structure-activity/property relationships and QSAR modeling of anti-amoebic activity of ctral derived amides, 4, 124.
Dods, L. R. and Donnelly, D. (2016) The peptide agonist-binding site of the glucagon-like peptide-1 (GLP-1) receptor based on site-directed mutagenesis and knowledge-based modelling. Bioscience Reports, 36.
Srivastava, S., Shree, P., & Tripathi, Y. B. (2017). Active phytochemicals of Pueraria tuberosa for DPP-IV inhibition: In silico and experimental approach. Journal of Diabetes & Metabolic Disorders, 16, 1–9.
Ogata, S., Misumi, Y., Tsuji, E., Takami, N., Oda, K., & Ikehara, Y. (1992). Identification of the active site residues in Dipeptidyl Peptidase IV by affinity labeling and site-directed mutagenesis. American Chemical Society, 31, 2582–2587.
Metzler, J. W., Yanchunas, J., Weigelt, C., Kish, K., Klei, H. E., Xie, D., Zhang, Y., Tamura, C. K. J., He, B., Hamann, G. L., Kirby, S. M., & Marcinkeviciene, J. (2008). Protein Science, Cold Spring Harbor Laboratory Press, San Diego, CA 92121. USA, 17, 2008.
Lexa, W. K., Dolghih, E. and Jacobson, P. M. (2014) A structure-based model for predicting serum albumin binding. PLOS One, 9.
Pereira, S. P. A., Banegas-Luna, A. J., & JPeña-García, J., Pérez-Sánchez, H. and Apostolides, Z. (2019). Evaluation of the anti-diabetic activity of some common herbs and spices: Providing new insights with inverse virtual screening. Molecules, 24, 1–42.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Saptadipa Paul. The first draft of the manuscript was written by Saptadipa Paul; Mala Majumdar commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, S., Majumdar, M. Multimode Assessment of Commercial Polyherbal Formulation: an In Vitro and In Silico Approach. Appl Biochem Biotechnol 195, 2261–2281 (2023). https://doi.org/10.1007/s12010-022-04064-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04064-7