Abstract
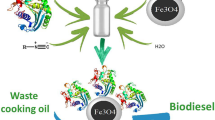
This study aimed to (i) prepare functionalized maghemite nanoparticles for immobilization of Candida rugosa lipase (CRL) by covalent binding, (ii) evaluate the application of the immobilized derivative in the hydrolysis of waste cooking oil (WCO) to fatty acids, and (iii) assess the potential of the hydrolyzed material for biodiesel production by hydroesterification. Maghemite (γFe2O3) obtained by precipitation of Fe3Cl2 with NH4OH served as an efficient support for covalent immobilization of CRL. Fourier-transform infrared spectroscopy and hydrolytic activity analysis indicated that CRL was covalently immobilized on the surface of the maghemite support. The derivative showed an activity of 166.62 ± 8 U g−1 in WCO hydrolysis at 40 °C and pH 6. Scanning electron microscopy revealed that, after lipase immobilization, nanoparticles became more dispersed, which is advantageous for biocatalysis reactions, as it increases the contact area with the substrate. WCO hydrolysis afforded 96 ± 0.2 wt% free fatty acids. In the second step, free fatty acids were subjected to chemical esterification with sulfuric acid, affording 94.4 ± 0.02 wt% fatty acid methyl esters (biodiesel). The findings of this study contribute to the field of biotechnology and may promote the development of enzymatic technologies for the synthesis of products of economic and social interest.








Similar content being viewed by others
Data Availability
Not applicable.
References
Remonatto, D., Ferrari, B. R., Bassan, J. C., Mussagy, C. U., de Carvalho Santos-Ebinuma, V., & de Paula, A. V. (2021). Utilization of clay materials as support for Aspergillus japonicus Lipase: An eco-friendly approach. Catalysts, 11(10), 1173. https://doi.org/10.3390/CATAL11101173
Bassan, N., Rodrigues, R. H., Monti, R., Tecelão, C., Ferreira-Dias, S., & Paula, A. V. (2019). Enzymatic modification of grapeseed (Vitis vinifera L.) oil aiming to obtain dietary triacylglycerols in a batch reactor. LWT, 99, 600–606. https://doi.org/10.1016/J.LWT.2018.05.013
Wancura, J. H. C., Rosset, D. V., Ugalde, G. A., Oliveira, J. V., Mazutti, M. A., Tres, M. V., & Jahn, S. L. (2019). Feeding strategies of methanol and lipase on eversa® transform-mediated hydroesterification for FAME production. The Canadian Journal of Chemical Engineering, 97(S1), 1332–1339. https://doi.org/10.1002/cjce.23404
dos Santos, L. K., Hatanaka, R. R., de Oliveira, J. E., & Flumignan, D. L. (2017). Experimental factorial design on hydroesterification of waste cooking oil by subcritical conditions for biodiesel production. Renewable Energy, 114, 574–580. https://doi.org/10.1016/J.RENENE.2017.07.066
Remonatto, D., Oliveira, J. V., Guisan, J. M., Oliveira, D., Ninow, J., & Fernandez-Lorente, G. (2022). Immobilization of eversa lipases on hydrophobic supports for ethanolysis of sunflower oil solvent-free. Applied Biochemistry and Biotechnology, 1–17. https://doi.org/10.1007/S12010-021-03774-8/FIGURES/5
Miotti, R. H., Jr., Cortez, D. V., & de Castro, H. F. (2022). Transesterification of palm kernel oil with ethanol catalyzed by a combination of immobilized lipases with different specificities in continuous two-stage packed-bed reactor. Fuel, 310, 122343. https://doi.org/10.1016/J.FUEL.2021.122343
da Silva Corrêa, L., Henriques, R. O., Rios, J. V., Lerin, L. A., de Oliveira, D., & Furigo, A. (2020). Lipase-catalyzed esterification of geraniol and citronellol for the synthesis of terpenic esters. Applied Biochemistry and Biotechnology, 190(2), 574–583. https://doi.org/10.1007/s12010-019-03102-1
de Meneses, A. C., Lerin, L. A., Araújo, P. H. H., Sayer, C., & de Oliveira, D. (2019). Benzyl propionate synthesis by fed-batch esterification using commercial immobilized and lyophilized Cal B lipase. Bioprocess and Biosystems Engineering, 42(10), 1625–1634. https://doi.org/10.1007/s00449-019-02159-w
Shuai, W., Das, R. K., Naghdi, M., Brar, S. K., & Verma, M. (2017). A review on the important aspects of lipase immobilization on nanomaterials. Biotechnology and Applied Biochemistry, 64(4), 496–508. https://doi.org/10.1002/bab.1515
Domínguez De María, P., Sánchez-Montero, J. M., Sinisterra, J. V., & Alcántara, A. R. (2006). Understanding Candida rugosa lipases: An overview. Biotechnology Advances, 24(2), 180–196. https://doi.org/10.1016/j.biotechadv.2005.09.003
Remonatto, D., Miotti Júnior, R. H., Monti, R., Bassan, J. C., & de Paula, A. V. (2022). Applications of immobilized lipases in enzymatic reactors: A review. Process Biochemistry. https://doi.org/10.1016/J.PROCBIO.2022.01.004
Poppe, J. K., Fernandez-Lafuente, R., Rodrigues, R. C., & Ayub, M. A. Z. (2015, September 1). Enzymatic reactors for biodiesel synthesis: Present status and future prospects. Biotechnology Advances. Elsevier Inc. https://doi.org/10.1016/j.biotechadv.2015.01.011
Filho, D. G., Silva, A. G., & Guidini, C. Z. (2019). Lipases: Sources, immobilization methods, and industrial applications. Applied Microbiology and Biotechnology, 103(18), 7399–7423. https://doi.org/10.1007/s00253-019-10027-6
Abdulla, R., & Ravindra, P. (2013). Immobilized Burkholderia cepacia lipase for biodiesel production from crude Jatropha curcas L. oil. Biomass and Bioenergy, 56, 8–13. https://doi.org/10.1016/J.BIOMBIOE.2013.04.010
Xie, W., & Huang, M. (2018). Immobilization of Candida rugosa lipase onto graphene oxide Fe3O4 nanocomposite: Characterization and application for biodiesel production. Energy Conversion and Management, 159, 42–53. https://doi.org/10.1016/J.ENCONMAN.2018.01.021
Xie, W., & Zang, X. (2018). Lipase immobilized on ionic liquid-functionalized magnetic silica composites as a magnetic biocatalyst for production of trans-free plastic fats. Food Chemistry, 257, 15–22. https://doi.org/10.1016/J.FOODCHEM.2018.03.010
Rajput, S., Pittman, C. U., & Mohan, D. (2016). Magnetic magnetite (Fe3O4) nanoparticle synthesis and applications for lead (Pb2+) and chromium (Cr6+) removal from water. Journal of Colloid and Interface Science, 468, 334–346. https://doi.org/10.1016/j.jcis.2015.12.008
Peng, X., Wang, Y., Tang, X., & Liu, W. (2011). Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles as selectivity-enhanced chemosensor for Hg(II). Dyes and Pigments, 91(1), 26–32. https://doi.org/10.1016/j.dyepig.2011.01.012
Remonatto, D., Miotti, R. H., Monti, R., Bassan, J. C., & de Paula, A. V. (2022). Applications of immobilized lipases in enzymatic reactors: A review. Process Biochemistry, 114, 1–20. https://doi.org/10.1016/J.PROCBIO.2022.01.004
Xie, W., & Wang, J. (2014). Enzymatic production of biodiesel from soybean oil by using immobilized lipase on Fe3O4/Poly(styrene-methacrylic acid) magnetic microsphere as a biocatalyst. Energy and Fuels, 28(4), 2624–2631. https://doi.org/10.1021/EF500131S/ASSET/IMAGES/LARGE/EF-2014-00131S_0012.JPEG
Xie, W., & Zang, X. (2016). Immobilized lipase on core-shell structured Fe3O4-MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard. Food Chemistry. https://doi.org/10.1016/j.foodchem.2015.09.009
Wu, W., Wu, Z., Yu, T., Jiang, C., & Kim, W.-S. (2015). Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Science and Technology of Advanced Materials, 16(2), 023501. https://doi.org/10.1088/1468-6996/16/2/023501
Lucena, G. N., dos Santos, C. C., Pinto, G. C., da Rocha, C. O., Brandt, J. V., de Paula, A. V., & Marques, R. F. C. (2019). Magnetic cross-linked enzyme aggregates (MCLEAs) applied to biomass conversion. Journal of Solid State Chemistry, 270, 58–70. https://doi.org/10.1016/J.JSSC.2018.11.003
Sheldon, R. A., Basso, A., & Brady, D. (2021). New frontiers in enzyme immobilisation: Robust biocatalysts for a circular bio-based economy. Chemical Society Reviews, 50(10), 5850–5862. https://doi.org/10.1039/D1CS00015B
Dunlop, D. J., & Özdemir, Ö. (1997). Rock magnetism: Fundamentals and frontiers. Cambridge Studies in Magnetism.
Zhong, L., Feng, Y., Wang, G., Wang, Z., Bilal, M., Lv, H., … Cui, J. (2020, June 1). Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. International Journal of Biological Macromolecules. Elsevier B.V. https://doi.org/10.1016/j.ijbiomac.2020.02.258
Gomes de Souza, F., Marins, J. A., Rodrigues, C. H. M., & Pinto, J. C. (2010). A magnetic composite for cleaning of oil spills on water. Macromolecular Materials and Engineering, 295(10), 942–948. https://doi.org/10.1002/mame.201000090
Hanefeld, U., Gardossi, L., & Magner, E. (2009). Understanding enzyme immobilisation. Chemical Society Reviews, 38(2), 453–468. https://doi.org/10.1039/B711564B
Trindade Ximenes, I. A., de Oliveira, P. C. O., Wegermann, C. A., & de Moraes, M. C. (2021). Magnetic particles for enzyme immobilization: A versatile support for ligand screening. Journal of Pharmaceutical and Biomedical Analysis, 204, 114286. https://doi.org/10.1016/J.JPBA.2021.114286
de Sousa, J. S., Cavalcanti-Oliveira, E. D. A., Aranda, D. A. G., & Freire, D. M. G. (2010). Application of lipase from the physic nut (Jatropha curcas L) to a new hybrid (enzyme/chemical) hydroesterification process for biodiesel production. Journal of Molecular Catalysis B: Enzymatic, 65(1–4), 133–137. https://doi.org/10.1016/j.molcatb.2010.01.003
Mendes, D. B., Silva, F. F. D., Guarda, P. M., Almeida, A. F., Oliveira, D. P. D., Morais, P. B., & Guarda, E. A. (2019). Lipolytic enzymes with hydrolytic and esterification activities produced by filamentous fungi isolated from decomposition leaves in an aquatic environment. Enzyme Research, 2019. https://doi.org/10.1155/2019/8182425
Barnebey, H. L., & Brown, A. C. (1948). Continuous fat splitting plants using the Colgate-Emery process. Journal of the American Oil Chemists’ Society, 25(3), 95–99. https://doi.org/10.1007/BF02579733
Pourzolfaghar, H., Abnisa, F., Daud, W. M. A. W., & Aroua, M. K. (2016). A review of the enzymatic hydroesterification process for biodiesel production. Renewable and Sustainable Energy Reviews, 61, 245–257. https://doi.org/10.1016/J.RSER.2016.03.048
Kulkarni, M. G., & Dalai, A. K. (2006). Waste cooking oil an economical source for biodiesel: A review. Industrial & Engineering Chemistry Research, 45(9), 2901–2913. https://doi.org/10.1021/ie0510526
Kumar, V., Jahan, F., Raghuwanshi, S., Mahajan, R. V., & Saxena, R. K. (2013). Immobilization of Rhizopus oryzae lipase on magnetic Fe3O4-chitosan beads and its potential in phenolic acids ester synthesis. Biotechnology and Bioprocess Engineering, 18(4), 787–795. https://doi.org/10.1007/s12257-012-0793-8
Vescovi, V., Kopp, W., Guisán, J. M., Giordano, R. L. C., Mendes, A. A., & Tardioli, P. W. (2016). Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino- glutaraldehyde spacer arms. Process Biochemistry, 51(12), 2055–2066. https://doi.org/10.1016/j.procbio.2016.09.016
Paula, A. V., Nunes, G. F. M., Santos, J. C., & de Castro, H. F. (2011). Interesterification of milkfat with soybean oil catalysed by Rhizopus oryzae lipase immobilised on SiO2-PVA on packed bed reactor. International Journal of Food Science & Technology, 46(10), 2124–2130. https://doi.org/10.1111/J.1365-2621.2011.02726.X
Tourinho, F. A., Depeyrot, J., Silva, G. J. da, & Lara, M. C. L. (1998). Electric double layered magnetic fluids (EDL-MF) based on spinel ferrite nanostructures [(M1-x+2Fex+3)]A [(Fe2-x+3 Mx+2)]BO4-2. Brazilian Journal of Physics. https://doi.org/10.1590/s0103-97331998000400016
American Oil Chemist Society. (2012). AOCS Ca 5a–40: Free fatty acids. American Oil Chemist Society.
Materials, A. S. for T. and. (2016). ASTM D6304–16e1: Standard test method for determination of water in petroleum products, lubricating oils, and additives by coulometric Karl Fischer titration. West Conshohocken, PA,: American Society for Testing and Materials. https://doi.org/10.1520/D6304-16E01
Associação Brasileira de Normas Técnicas. (2015). ABNT NBR 15908:2015: BIODIESEL - Determinação do glicerol livre, mono-, di-, triacilgliceróis e glicerol total por cromatografia gasosa. Rio de Janeiro, RJ: Associação Brasileira de Normas Técnicas.
ISO International standard. (1990). ET ISO 5508:1990: Animal and vegetable fats and oils - Analysis by gas chromatography of methyl esters of fatty acids. Switzerland: ISO International standard.
dos Santos, L. K., Botti, R. F., de Mello Innocentini, M. D., Marques, R. F. C., Colombo, P., de Paula, A. V., & Flumignan, D. L. (2021). 3D printed geopolymer: An efficient support for immobilization of Candida rugosa lipase. Chemical Engineering Journal, 414, 128843. https://doi.org/10.1016/J.CEJ.2021.128843
Hassan, S. Z., & Vinjamur, M. (2014). Parametric effects on kinetics of esterification for biodiesel production: A Taguchi approach. Chemical Engineering Science, 110, 94–104. https://doi.org/10.1016/j.ces.2013.11.049
Hao, Y., Chen, Y., Xia, H., & Gao, Q. (2019). Surface chemical functionalization of starch nanocrystals modified by 3-aminopropyl triethoxysilane. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2018.12.200
Liang, Y., Huang, J., Zang, P., Kim, J., & Hu, W. (2014). Molecular layer deposition of APTES on silicon nanowire biosensors: Surface characterization, stability and pH response. Applied Surface Science, 322, 202–208. https://doi.org/10.1016/j.apsusc.2014.10.097
Lehninger N., D., & Cox M., M. (2009). Principios de Bioquímica. Lehninger - Principios de Bioquimica 5ed.
Wang, X. Y., Jiang, X. P., Li, Y., Zeng, S., & Zhang, Y. W. (2015). Preparation Fe3O4@ chitosan magnetic particles for covalent immobilization of lipase from Thermomyceslanuginosus. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2015.01.020
Ozyilmaz, E., Bayrakci, M., & Yilmaz, M. (2016). Improvement of catalytic activity of Candida rugosa lipase in the presence of calix[4]arene bearing iminodicarboxylic/phosphonic acid complexes modified iron oxide nanoparticles. Bioorganic Chemistry.https://doi.org/10.1016/j.bioorg.2015.12.001
Guidini, C. Z., Fischer, J., Santana, L. N. S., Cardoso, V. L., & Ribeiro, E. J. (2010). Immobilization of Aspergillus oryzae β-galactosidase in ion exchange resins by combined ionic-binding method and cross-linking. Biochemical Engineering Journal, 52(2–3), 137–143. https://doi.org/10.1016/J.BEJ.2010.07.013
Guisan, J. M., López-Gallego, F., Bolivar, J. M., Rocha-Martín, J., & Fernandez-Lorente, G. (2020). The science of enzyme immobilization. In Methods in Molecular Biology (Vol. 2100, pp. 1–26). Humana Press Inc. https://doi.org/10.1007/978-1-0716-0215-7_1
Pavia, D., Lampman, G., Kriz, G., & Vyvyan, J. (2012). Introdução à espectroscopia.Cengage Learning. https://doi.org/10.1590/S0100-40422007000700048
Ball, D. W. (2009). The basics of spectroscopy. The Basics of Spectroscopy. DOI, 10(1117/3), 422981.
Izrael Živković, L. T., Živković, L. S., Babić, B. M., Kokunešoski, M. J., Jokić, B. M., & Karadžić, I. M. (2015). Immobilization of Candida rugosa lipase by adsorption onto biosafe meso/macroporous silica and zirconia. Biochemical Engineering Journal, 93, 73–83. https://doi.org/10.1016/j.bej.2014.09.012
Ali, Z., Tian, L., Zhang, B., Ali, N., Khan, M., & Zhang, Q. (2017). Synthesis of paramagnetic dendritic silica nanomaterials with fibrous pore structure (Fe3O4@KCC-1) and their application in immobilization of lipase from: Candida rugosa with enhanced catalytic activity and stability. New Journal of Chemistry, 41(16), 8222–8231. https://doi.org/10.1039/c7nj01912b
Brígida, A. I. S., Calado, V. M. A., Gonçalves, L. R. B., & Coelho, M. A. Z. (2010). Effect of chemical treatments on properties of green coconut fiber. Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2009.10.005
Silva, M. O. da, Carneiro, M. L. B., Siqueira, J. L. N., Báo, S. N., & Souza, A. R. de. (2017). Development of a promising antitumor compound based on rhodium(II) succinate associated with iron oxide nanoparticles coated with lauric acid/albumin hybrid: Synthesis, colloidal stability and cytotoxic effect in breast carcinoma cells. Journal of Nanoscience and Nanotechnology. https://doi.org/10.1166/jnn.2018.15021
Calmon, M. F., de Souza, A. T., Candido, N. M., Raposo, M. I. B., Taboga, S., Rahal, P., & Nery, J. G. (2012). A systematic study of transfection efficiency and cytotoxicity in HeLa cells using iron oxide nanoparticles prepared with organic and inorganic bases. Colloids and Surfaces B: Biointerfaces. https://doi.org/10.1016/j.colsurfb.2012.05.026
Millan, A., Palacio, F., Falqui, A., Snoeck, E., Serin, V., Bhattacharjee, A., … Gilbert, I. (2007). Maghemite polymer nanocomposites with modulated magnetic properties.Acta Materialia. https://doi.org/10.1016/j.actamat.2006.11.020
Cornell, R. M., & Schwertmann, U. (2003). The iron oxides: Structure, properties, reactions, occurrences and uses: second ed. WILEY-VCH GmbH&Co. KGaA. https://doi.org/10.1002/3527602097
Badoei-dalfard, A., Malekabadi, S., Karami, Z., & Sargazi, G. (2019). Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renewable Energy, 141, 874–882. https://doi.org/10.1016/j.renene.2019.04.061
Barriuso, J., Vaquero, M. E., Prieto, A., & Martínez, M. J. (2016). Structural traits and catalytic versatility of the lipases from the Candida rugosa-like family: A review. Biotechnology Advances, 34(5), 874–885. https://doi.org/10.1016/j.biotechadv.2016.05.004
Li, G., Chen, Y., Fang, X., Su, F., Xu, L., & Yan, Y. (2018). Identification of a hot-spot to enhance: Candida rugosa lipase thermostability by rational design methods. RSC Advances, 8(4), 1948–1957. https://doi.org/10.1039/c7ra11679a
Hadadi, M., & Habibi, A. (2019). Candida rugosa lipase immobilized on functionalized magnetic Fe3O4 nanoparticles as a sustainable catalyst for production of natural epoxides. Chemical Papers, 73(8), 1917–1929. https://doi.org/10.1007/S11696-019-00741-W/TABLES/3
Jafarian, F., Bordbar, A. K., Zare, A., & Shams-Solari, E. (2020). An enzymatic performance for a new swift magnetically detachable bio-conjugate of Candida rugosa lipase with modified Fe3O4–graphene oxide nanocomposite. Journal of the Iranian Chemical Society, 17(2), 367–382. https://doi.org/10.1007/S13738-019-01773-5/FIGURES/15
Talukder, M. M. R., Wu, J. C., & Chua, L. P. L. (2010). Conversion of waste cooking oil to biodiesel via enzymatic hydrolysis followed by chemical esterification. Energy and Fuels, 24(3), 2016–2019. https://doi.org/10.1021/ef9011824
Xie, W., & Huang, M. (2020). Fabrication of immobilized Candida rugosa lipase on magnetic Fe3O4-poly(glycidyl methacrylate-co-methacrylic acid) composite as an efficient and recyclable biocatalyst for enzymatic production of biodiesel. Renewable Energy, 158, 474–486. https://doi.org/10.1016/j.renene.2020.05.172
Katiyar, M., Abida, K., & Ali, A. (2021). Candida rugosa lipase immobilization over SBA-15 to prepare solid biocatalyst for cotton seed oil transesterification. Materials Today: Proceedings, 36, 763–768. https://doi.org/10.1016/J.MATPR.2020.06.061
Ma, C., Zhang, Y., Yang, C., Zhang, Y., Zhang, M., & Tang, J. (2022). Cetyl trimethyl ammonium bromide-activated lipase from Aspergillus oryzae immobilized with Cu3(PO4)2⋅3H2O via biomineralization for hydrolysis of olive oil. LWT, 159, 113204. https://doi.org/10.1016/J.LWT.2022.113204
Ozyilmaz, E., Ascioglu, S., & Yilmaz, M. (2021). Preparation of one-pot immobilized lipase with Fe3O4 nanoparticles into metal-organic framework for enantioselective hydrolysis of (R, S)-naproxen methyl ester. ChemCatChem, 13(16), 3687–3694. https://doi.org/10.1002/CCTC.202100481
Ou, J., Yuan, X., Liu, Y., Zhang, P., Xu, W., & Tang, K. (2021). Lipase from pseudomonas cepacia immobilized into ZIF-8 as bio-catalyst for enantioselective hydrolysis and transesterification. Process Biochemistry, 102, 132–140. https://doi.org/10.1016/J.PROCBIO.2020.12.017
Pinto, G. C., Brandt, J. V., Piazza, R. D., dos Santos, C. C., Lucena, G. N., de Paula, A. V., & Marques, R. F. C. (2021). Magnetic graphene oxide as a carrier for lipases immobilization: An approach for hydrolysis of olive oil emulsion. ECS Journal of Solid State Science and Technology, 10(6), 065008. https://doi.org/10.1149/2162-8777/AC054A
Jafarian, F., Bordbar, A.-K., Razmjou, A., & Zare, A. (2020). The fabrication of a high performance enzymatic hybrid membrane reactor (EHMR) containing immobilized Candida rugosa lipase (CRL) onto graphene oxide nanosheets-blended polyethersulfone membrane. Journal of Membrane Science, 613, 118435. https://doi.org/10.1016/j.memsci.2020.118435
Shivaprasad, P., Jones, M. D., Frith, P., & Emanuelsson, E. A. C. (2020). Investigating the effect of increasing cloth size and cloth number in a spinning mesh disc reactor (SMDR): A study on the reactor performance. Chemical Engineering and Processing - Process Intensification, 147, 107780. https://doi.org/10.1016/j.cep.2019.107780
Castiglioni, G. Z., Bettio, G., Matte, C. R., Jacques, R. A., Dos Santos Polidoro, A., Rosa, C. A., & Ayub, M. A. Z. (2020). Production of volatile compounds by yeasts using hydrolysed grape seed oil obtained by immobilized lipases in continuous packed-bed reactors.Bioprocess and Biosystems Engineering, 1–12. https://doi.org/10.1007/s00449-020-02334-4
Bavaro, T., Benucci, I., Pedrali, A., Marrubini, G., Esti, M., Terreni, M., & Ubiali, D. (2020). Lipase-mediated hydrolysis of hempseed oil in a packed-bed reactor and in-line purification of PUFA as mono- and diacylglycerols. Food and Bioproducts Processing, 123, 345–353. https://doi.org/10.1016/j.fbp.2020.07.009
Zare, A., Bordbar, A. K., Razmjou, A., & Jafarian, F. (2019). The immobilization of Candida rugosa lipase on the modified polyethersulfone with MOF nanoparticles as an excellent performance bioreactor membrane. Journal of Biotechnology, 289, 55–63. https://doi.org/10.1016/j.jbiotec.2018.11.011
Xu, J., Liu, C., Wang, M., Shao, L., Deng, L., Nie, K., & Wang, F. (2017). Rotating packed bed reactor for enzymatic synthesis of biodiesel. Bioresource Technology, 224, 292–297. https://doi.org/10.1016/j.biortech.2016.10.045
Gupta, S. (2016). Comparative study on hydrolysis of oils by lipase immobilized biocatalytic PS membranes using biphasic enzyme membrane reactor. Journal of Environmental Chemical Engineering, 4(2), 1797–1809. https://doi.org/10.1016/j.jece.2016.03.007
Hou, C., Qi, Z., & Zhu, H. (2015). Preparation of core–shell magnetic polydopamine/alginate biocomposite for Candida rugosa lipase immobilization. Colloids and Surfaces B: Biointerfaces, 128, 544–551. https://doi.org/10.1016/J.COLSURFB.2015.03.007
Funding
This research used resources from the Center for Monitoring and Research of the Quality of Fuels, Biofuels, Crude Oil and Derivatives, which is supported by the FUNDUNESP. This study was financed in part by the São Paulo Research Foundation (FAPESP, grant nos. 2018/09904–3 and 2020/09592–1) and the Brazilian National Council for Scientific and Technological Development (CNPq, process no. 28/2018, grant no. 424931/2018–4).
Author information
Authors and Affiliations
Contributions
All authors have read and agreed to the published version of the manuscript. Otávio Domingues: Investigation and writing (original draft preparation). Daniela Remonatto: Investigation and writing (original draft preparation). Letícia K. dos Santos: Writing (review and editing) and validation. Julián Paul Martínez Galán: Conceptualization, writing (review and editing), and validation. Danilo Luiz Flumignan: Conceptualization, investigation, and supervision. Ariela V. de Paula: Conceptualization, investigation, supervision, and resources.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals.
Consent to Participate
All authors agree mutually with the participation and publication of this work and declare that this is an original research.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Domingues, O., Remonatto, D., dos Santos, L.K. et al. Evaluation of Candida rugosa Lipase Immobilized on Magnetic Nanoparticles in Enzymatic/Chemical Hydroesterification for Biodiesel Production. Appl Biochem Biotechnol 194, 5419–5442 (2022). https://doi.org/10.1007/s12010-022-04046-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04046-9