Abstract
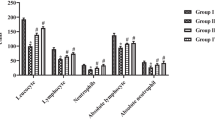
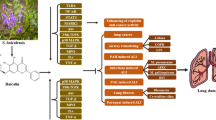
Lung cancer, one of the most often diagnosed malignancies, is the top cause of death in both men and women globally. In both developed and emerging countries, high incidences of cancer are becoming a huge health burden. Natural resources, including plants, have always been a possible source of lead compounds in the identification of optimal medications for cancer treatment, with natural resources accounting for around half of all anticancer drugs. Ruscogenin, a natural saponin, is a major component of Radix Ophiopogon japonicus with a well-established anticancer activity. In this study, the anticancer potential of ruscogenin against a B(a)P-challenged lung cancer model in mice was assessed. The mice were categorized into four groups: group I was as the control group, group II mice were challenged with B(a)P, group III rodents were treated with ruscogenin prior to challenge with B(a)P, and group IV rodents were treated with ruscogenin after B(a)P administration. Tumor incidence was calculated, and the following parameters were analyzed: body weight, lung weight, immunoglobulin (Ig) levels (IgG, IgA, and IgM), key marker enzymes, and proinflammatory cytokines in both treated and control mice. Lung tissues were analyzed via histopathological analysis. According to our results, all the markers that favor the growth of cancer were increased in the lung cancer group. After administration of ruscogenin, all the markers returned to their original levels, revealing the anticancer potential of ruscogenin.









Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- Ig:
-
Immunoglobulin
- B(a)P:
-
Benzo[a]pyrene
- TCM:
-
Traditional Chinese medicine
- GGT:
-
Gamma-glutamyl transferase
- ADA:
-
Adenosine deaminase
- LDH:
-
Lactate dehydrogenase
- AAH:
-
Aryl hydrocarbon hydroxylase
- 5′-NT:
-
5′-Nucleotidase
- CEA:
-
Carcinoembryonic antigen
- ICDH:
-
Isocitrate dehydrogenase
- SDH:
-
Succinate dehydrogenase
References
Wang, X., Veeraraghavan, V. P., Mohan, S. K., & Lv, F. (2021). Anticancer and immunomodulatory effect of rhaponticin on benzo (a) pyrene-induced lung carcinogenesis and induction of apoptosis in A549 cells. Saudi Journal of Biological Sciences., 28(8), 4522–4531.
World Health Organization (WHO). (2021). Global cancer observatory: Cancer today. Available from: http://gco.iarc.fr.
Khan, A., Alhumaydhi, F. A., Alwashmi, A. S., Allemailem, K. S., Alsahli, M. A., Alrumaihi, F. A., Almatroudi, A., Mobark, M. A., Mousa, A., & Khan, M. A. (2020). Diallyl sulfide-mediated modulation of the fatty acid synthase (FASN) leads to cancer cell death in bap-induced lung carcinogenesis in Swiss mice. Journal of Inflammation Research., 13, 1075.
Barta, J. A., Powell, C. A., & Wisnivesky, J. P. (2019). Global epidemiology of lung cancer. Annals of Global Health., 85(1), 8. https://doi.org/10.5334/aogh.2419.
Liu, J., Yang, Z., Kong, Y., He, Y., Xu, Y., & Cao, X. (2019). Antitumor activity of alantolactone in lung cancer cell lines NCI-H1299 and Anip973. Journal of Food Biochemistry., 43(9), e12972.
Ramalingam, V., & Rajaram, R. (2018). Enhanced antimicrobial, antioxidant and anticancer activity of Rhizophora apiculata: An experimental report. 3 Biotech., 8(4), 1–3.
Wang, C. C., Yuan, J. R., Wang, C. F., Yang, N., Chen, J., Liu, D., Song, J., Feng, L., Tan, X. B., & Jia, X. B. (2017). Anti-inflammatory effects of Phyllanthus emblica L on benzopyrene-induced precancerous lung lesion by regulating the IL-1β/miR-101/Lin28B signaling pathway. Integrative Cancer Therapies., 16(4), 505–515.
Xiao, B., Lin, D., Zhang, X., Zhang, M., & Zhang, X. (2016). TTF1, in the form of nanoparticles, inhibits angiogenesis, cell migration and cell invasion in vitro and in vivo in human hepatoma through STAT3 regulation. Molecules, 21(11), 1507.
Shendge, A. K., Sarkar, R., & Mandal, N. (2020). Potent anti-inflammatory Terminalia chebula fruit showed in vitro anticancer activity on lung and breast carcinoma cells through the regulation of Bax/Bcl-2 and caspase-cascade pathways. Journal of Food Biochemistry., 44(12), e13521.
Hua, H., Zhu, Y., & Song, Y. H. (2018). Ruscogenin suppressed the hepatocellular carcinoma metastasis via PI3K/Akt/mTOR signaling pathway. Biomedicine & Pharmacotherapy., 101, 115–122.
Khojasteh, A., Sanchez-Muñoz, R., Moyano, E., Bonfill, M., Cusido, R. M., Eibl, R., & Palazon, J. (2019). Biotechnological production of ruscogenins in plant cell and organ cultures of Ruscus aculeatus. Plant Physiology and Biochemistry., 141, 133–141.
Song, Z., Xiang, X., Li, J., Deng, J., Fang, Z., Zhang, L., & Xiong, J. (2020). Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncology Reports., 43(2), 516–524.
Ma, A. Z. (2014). Mechanism of ruscogenin on suppressing pulmonary A549 and H460 cells. Nanjing University of Chinese Medicine.
Tennant, B., Baldwin, B. H., Braun, R. K., Norcross, N. L., & Sandholm, M. (1979). Use of the glutaraldehyde coagulation test for deduction of hypogammaglobulinemia in neonatal calves. Journal of the American Veterinary Medical Association, 174, 848–853.
Satpathy, P. K., Dutta, N. K., Mishra, P. R., & Kai, B. (1996). Glutaraldehyde coagulation test: Standard curve and its applications to detect gammaglobulin level in kids. The Indian Veterinary Journal, 73(3), 257–260.
Bergmeyer, H.U. (1984). Principles of enzymatic analysis. In: Methods of enzymatic analysis. Verlag Chemie
Buening, M. K., Chang, R. L., Huang, M. T., Fortner, J. G., Wood, A. W., & Conney, A. H. (1981). Activation and inhibition of benzo (a) pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally occurring flavonoids. Cancer Research, 41(1), 67–72.
Orlowski, M., & Meister, A. (1965). Isolation of γ-glutamyl transpeptidase from hog kidney. Journal of Biological Chemistry, 240, 338–347.
Luly, P., Barnabei, O., & Tria, E. (1972). Hormonal control in vitro of plasma membrane-bound (Na+− K+)-ATPase of rat liver. Biochimica Et Biophysica Acta, 282, 447–452.
King, C. (1965). The transferases-alanine and aspartate transaminases. In D. Van (Ed.), Practical Clinical Enzymology (pp. 121–138). Nostrand Company Ltd.
Wharton, D. C., & Tzagoloff, A. (1967). Methods in Enzymology (pp. 245–250). Academic Press.
Omura, T., & Sato, R. (1964). The carbon monoxide-binding pigment of liver microsomes: I. Evidence for its hemoprotein nature. Journal of Biological Chemistry, 239(7), 2370–2378.
Benson, A. M., Hunkeler, M. J., & Talalay, P. (1980). Increase of NAD (P) H: Quinone reductase by dietary antioxidants: Possible role in protection against carcinogenesis and toxicity. Proceedings of the National Academy of Sciences, 77(9), 5216–5220.
Luquita, M. G., Sánchez Pozzi, E. J., Catania, V. A., & Mottino, A. D. (1994). Analysis of p-nitrophenol glucuronidation in hepatic microsomes from lactating rats. Biochemical Pharmacology, 47(7), 1179–1185.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249(22), 7130–7139.
Naveenkumar, C., Asokkumar, S., Raghunandhakumar, S., Jagan, S., Anandakumar, P., Augustine, T. A., Kamaraj, S., & Devaki, T. (2012). Potent antitumor and antineoplastic efficacy of baicalein on benzo (a) pyrene-induced experimental pulmonary tumorigenesis. Fundamental and Clinical Pharmacology, 2(2), 259–270.
Johnson, D., & Lardy, H. (1967). Isolation of liver or kidney mitochondria. Methods in Enzymology, 10, 94–96.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Reed, L. J., & Mukherjee, B. B. (1969). Methods in enzymology (pp. 55–61). Academic Press.
Slater, E. C., & Borner, W. D. (1952). The effect of fluoride on succinic oxidase system. The Biochemical Journal, 52, 185–196.
Mehler, A. H., & Kornberg, A. (1948). The enzymatic mechanism of oxidation-reductions between malate or isocitrate and pyruvate. Journal of Biological Chemistry, 174, 961–977.
Birchmachin, M. A., Briggs, H. L., Saborido, A. A., Bindoff, L. A., & Turnbull, D. M. (1994). An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochemical Medicine and Metabolic Biology, 51(1), 35–42.
Krähenbühl, S., Talos, C., Wiesmann, U., & Hoppel, C. L. (1994). Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clinica Chimica Acta, 230(2), 177–187.
Capaldi, R. A., Maruschi, M. F., & Taanman, J. W. (1995). Methods of Biochemical Analysis, 2, 427–434.
Cicko, S., Lucattelli, M., Muller, T., Lommatzsch, M., De Cunto, G., et al. (2010). Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. The Journal of Immunology, 185, 688–697.
Hu, X., Geetha, R. V., Surapaneni, K. M., Veeraraghavan, V. P., Chinnathambi, A., Alahmadi, T. A., Manikandan, V., & Manokaran, K. (2021). Lung cancer induced by benzo(A)pyrene: Chemoprotective effect of sinapic acid in Swiss albino mice. Saudi Journal of Biological Sciences, 28(12), 7125–7133.
Arumugam, P., Arunkumar, K., Sivakumar, L., Murugan, M., & Murugan, K. (2019). Anticancer effect of fucoidan on cell proliferation, cell cycle progression, genetic damage and apoptotic cell death in HepG2 cancer cells. Toxicology Reports., 6, 556–563.
Gong, C., Qi, L., Huo, Y., Zhang, S., Ning, X., Bai, L., & Wang, Z. (2019). Anticancer effect of Limonin against benzo (a) pyrene-induced lung carcinogenesis in Swiss albino mice and the inhibition of A549 cell proliferation through apoptotic pathway. Journal of Biochemical and Molecular Toxicology., 33(12), e22374.
Hassan, S. K., Mousa, A. M., El-Sammad, N. M., Abdel-Halim, A. H., Khalil, W. K., Elsayed, E. A., Anwar, N., Linscheid, M. W., Moustafa, E. S., Hashim, A. N., & Nawwar, M. (2019). Antitumor activity of Cuphea ignea extract against benzo (a) pyrene-induced lung tumorigenesis in Swiss Albino mice. Toxicology Reports., 6, 1071–1085.
Velli, S. K., Sundaram, J., Murugan, M., Balaraman, G., & Thiruvengadam, D. (2019). Protective effect of vanillic acid against benzo (a) pyrene induced lung cancer in Swiss albino mice. Journal of Biochemical and Molecular Toxicology., 33(10), e22382.
Yoon, S. H. (2014). Immunotherapy for non-small cell lung cancer. Tuberculosis and Respiratory Diseases., 77(3), 111–115.
Singh, N., Baby, D., Rajguru, J. P., Patil, P. B., Thakkannavar, S. S., & Pujari, V. B. (2019). Inflammation and cancer. Annals of African Medicine., 18(3), 121.
Gào, X., Xuan, Y., Benner, A., Anusruti, A., Brenner, H., & Schöttker, B. (2019). Nitric oxide metabolites and lung cancer incidence: A matched case-control study nested in the ESTHER cohort. Oxidative Medicine and Cellular Longevity., 2019, 6470950. https://doi.org/10.1155/2019/6470950.
Zhao, P., & Zhang, Z. (2018). TNF-α promotes colon cancer cell migration and invasion by upregulating TROP-2. Oncology letters., 15(3), 3820–3827.
Kasala, E. R., Bodduluru, L. N., Barua, C. C., Sriram, C. S., & Gogoi, R. (2015). Benzo (a) pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacological Reports., 67(5), 996–1009.
Rao, P. S., Midde, N., Miller, D., Chauhan, S., Kumar, A., & Kumar, S. (2015). Diallyl sulfide: Potential use in novel therapeutic interventions in alcohol, drugs, and disease mediated cellular toxicity by targeting cytochrome P450 2E1. Current Drug Metabolism., 16(6), 486–503.
Miao, P., Sheng, S., Sun, X., Liu, J., & Huang, G. (2013). Lactate dehydrogenase A in cancer: A promising target for diagnosis and therapy. IUBMB Life, 65(11), 904–910.
Xie, H., Hanai, J. I., Ren, J. G., Kats, L., Burgess, K., Bhargava, P., Signoretti, S., Billiard, J., Duffy, K. J., Grant, A., & Wang, X. (2014). Targeting lactate dehydrogenase-a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metabolism., 19(5), 795–809.
Beauchemin, N., & Arabzadeh, A. (2013). Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer and Metastasis Reviews., 32(3), 643–671.
Thirunavukkarasu, C., Prince Vijeya Singh, J., Selvendiran, K., & Sakthisekaran, D. (2001). Chemopreventive efficacy of selenium against N-nitrosodiethylamine-induced hepatoma in albino rats. Cell Biochemistry and Function: Cellular Biochemistry and its Modulation by Active Agents or Disease., 19(4), 265–71.
Author information
Authors and Affiliations
Contributions
Jun Zhao, Bangzhi He, Vidya Devanathadesikan Seshadri, and Shaohua Xu contributed equally in working and drafting the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors have their consent to participate.
Consent to Publish
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., He, B., Seshadri, V.D. et al. Anticancer Effect of Ruscogenin in B(a)P-Induced Lung Cancer in Mice via Modulation of Proinflammatory Cytokines and Mitochondrial Enzymes. Appl Biochem Biotechnol 194, 5862–5877 (2022). https://doi.org/10.1007/s12010-022-04042-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04042-z