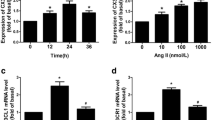
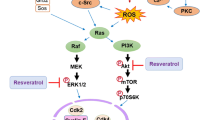
Abstract
Vascular smooth muscle cells (VSMCs) are associated with differentiated, organized, and contractile phenotype under the effect of various types of physiological conditions those are associated with migratory, proliferative, and synthetic phenotype under the effect of various types of stimuli, which dysfunction drives many cardiovascular diseases. Abnormal cell proliferation and invasion of VSMCs are among the primary causes of hypertension. Apatinib is a small-molecule tyrosine kinase inhibitor (TKI) that highly selectively binds to and strongly inhibits VEGFR-2. Previous studies have confirmed that the TKIs can raise blood pressure through RhoA/ROCK pathway. LARG is a key gene in the RhoA/ROCK pathway and plays a critical role in the continuous vasoconstriction function because it regulates part of signal transduction in VSMCs. In this study, an in vitro experiment was conducted to observe that apatinib caused dysfunction of MOVAS cells through the RhoA/ROCK signalling pathway and Y27632, a nonspecific ROCK inhibitor, and knockout of LARG gene can improve the proliferation, antiapoptosis, oxidative stress, and mitochondrial autophagy of apatinib-induced MOVAS cells. These findings suggest that activation of the RhoA/ROCK signalling pathway could be the underlying mechanism of apatinib-induced dysfunction of MOVAS cells, while ROCK inhibitor and knockout of LARG gene have potential therapeutic value.







Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- RhoA:
-
Rho-associated
- ROCK:
-
Rho-associated coiled-coil domain-containing protein kinase
- LARG:
-
Leukaemia-associated Rho-GEF
- BP:
-
Blood pressure
- TKIs:
-
Tyrosine kinase inhibitors
- VEGFR-2:
-
Vascular endothelial growth factor receptor-2
- VSMCs:
-
Vascular smooth muscle cells
- MYPT1:
-
Myosin phosphatase target-1
- MLC:
-
Myosin light chain
- MLCP:
-
Myosin light chain phosphatase
- CCK-8:
-
Cell counting kit-8
- shRNA:
-
RNA-short hairpin RNA
- ET-1:
-
Endothelin-1
- eNOS:
-
Endothelial nitric oxide synthase
- iNOS:
-
Inducible nitric oxide synthase
- a-SMA:
-
Alpha smooth muscle actin
- LC3:
-
MAP1LC3
- ROS:
-
Photo-activated reactive oxygen species
- Bax:
-
BCL2-Associated X
- Bcl2:
-
B cell lymphoma/leukaemia-2
References
Liu, K., Ren, T., Huang, Y., et al. (2017). Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death & Disease, 8, e3015.
Peng, S., Zhang, Y., Peng, H., et al. (2016). Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib. Cancer Letters, 373, 193–202.
Huang, M., Huang, B., Li, G., et al. (2018). Apatinib affect VEGF-mediated cell proliferation, migration, invasion via blocking VEGFR2/RAF/MEK/ERK and PI3K/AKT pathways in cholangiocarcinoma cell. BMC Gastroenterology, 18, 169.
Chu, L., Chen, Y., Liu, Q., et al. (2021). A Phase II study of apatinib in patients with chemotherapy-refractory esophageal squamous cell carcinoma (ESO-Shanghai 11). The Oncologist, 26, e925–e935.
Zhao, L., Peng, Y., He, S., et al. (2021). Apatinib induced ferroptosis by lipid peroxidation in gastric cancer. Gastric Cancer, 24, 642–654.
Xu, J., Zhang, Y., Jia, R., et al. (2019). Anti-PD-1 Antibody SHR-1210 Combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: An open-label, dose escalation and expansion study. Clinical Cancer Research, 25, 515–523.
Li, J., Qin, S., Xu, J., et al. (2013). Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: Results from a randomized, placebo-controlled, parallel-arm, phase II trial. Journal of Clinical Oncology, 31, 3219–3225.
Li, C., Ma, L., Wang, Q., et al. (2022). Rho kinase inhibition ameliorates vascular remodeling and blood pressure elevations in a rat model of apatinib-induced hypertension. Journal of Hypertension, 40, 675–684.
Wang, W., He, Q., Zhang, H., et al. (2021). A narrative review on the interaction between genes and the treatment of hypertension and breast cancer. Ann Transl Med, 9, 894.
Huang, C. H., Ciou, J. S., Chen, S. T., et al. (2016). Identify potential drugs for cardiovascular diseases caused by stress-induced genes in vascular smooth muscle cells. PeerJ, 4, e2478.
Tykocki, N. R., Boerman, E. M., & Jackson, W. F. (2017). Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Comprehensive Physiology, 7, 485–581.
Zhang, W., Chen, S., Zhang, Z., et al. (2017). FAM3B mediates high glucose-induced vascular smooth muscle cell proliferation and migration via inhibition of miR-322-5p. Science and Reports, 7, 2298.
Seccia, T. M., Rigato, M., Ravarotto, V., & Calò, L. A. (2020). ROCK (RhoA/Rho Kinase) in cardiovascular-renal pathophysiology: A review of new advancements. Journal of Clinical Medicine, 9(5), 1328. https://doi.org/10.3390/jcm9051328
Ito, M., Nakano, T., Erdodi, F., et al. (2004). Myosin phosphatase: Structure, regulation and function. Molecular and Cellular Biochemistry, 259, 197–209.
Chiu, W. C., Chiang, J. Y., & Chiang, F. T. (2021). Small chemical compounds Y16 and Rhosin can inhibit calcium sensitization pathway in vascular smooth muscle cells of spontaneously hypertensive rats. Journal of the Formosan Medical Association, 120, 1863–1868.
Syrovatkina, V., Alegre, K. O., Dey, R., et al. (2016). Regulation, signaling, and physiological functions of G-proteins. Journal of Molecular Biology, 428, 3850–3868.
Lankhorst, S., Severs, D., Markó, L., et al. (2017). Salt sensitivity of angiogenesis inhibition-induced blood pressure rise: Role of interstitial sodium accumulation? Hypertension, 69, 919–926.
Wirth, A., Benyó, Z., Lukasova, M., et al. (2008). G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nature Medicine, 14, 64–68.
Kitzing, T. M., Sahadevan, A. S., Brandt, D. T., et al. (2007). Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes & Development, 21, 1478–1483.
Goulimari, P., Knieling, H., Engel, U., et al. (2008). LARG and mDia1 link Galpha12/13 to cell polarity and microtubule dynamics. Molecular Biology of the Cell, 19, 30–40.
Thompson, W. R., Yen, S. S., Uzer, G., et al. (2018). LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage. Bone, 107, 172–180.
Banerjee, P., Harada, H., Tassew, N. G., et al. (2016). ϒ-secretase and LARG mediate distinct RGMa activities to control appropriate layer targeting within the optic tectum. Cell Death and Differentiation, 23, 442–453.
Chen, J., Deng, S., Zhang, Y., et al. (2021). Apatinib enhances the anti-tumor effect of paclitaxel via the PI3K/p65/Bcl-xl pathway in triple-negative breast cancer. Ann Transl Med, 9, 1001.
Krausgruber, T., Blazek, K., Smallie, T., et al. (2011). IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature Immunology, 12, 231–238.
Wang, X. Y., Mo, D., Tian, W., et al. (2019). Inhibition of RhoA/ROCK signaling pathway ameliorates hypoxic pulmonary hypertension via HIF-1α-dependent functional TRPC channels. Toxicology and Applied Pharmacology, 369, 60–72.
Xu, Y., Cui, K., Li, J., et al. (2020). Melatonin attenuates choroidal neovascularization by regulating macrophage/microglia polarization via inhibition of RhoA/ROCK signaling pathway. Journal of Pineal Research, 69, e12660.
Amano, M., Nakayama, M., & Kaibuchi, K. (2010). Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken), 67, 545–554.
Bishop, A. L., & Hall, A. (2000). Rho GTPases and their effector proteins. The Biochemical Journal, 348(Pt 2), 241–255.
Raftopoulou, M., & Hall, A. (2004). Cell migration: Rho GTPases lead the way. Developmental Biology, 265, 23–32.
Zhang, X., Ye, P., Wang, D., et al. (2019). Involvement of RhoA/ROCK signaling in Aβ-induced chemotaxis, cytotoxicity and inflammatory response of microglial BV2 cells. Cellular and Molecular Neurobiology, 39, 637–650.
Frismantiene, A., Philippova, M., Erne, P., et al. (2018). Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cellular Signalling, 52, 48–64.
Kuntic, M., Oelze, M., Steven, S., et al. (2020). Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: Evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). European Heart Journal, 41, 2472–2483.
Lankhorst, S., Saleh, L., Danser, A. J., et al. (2015). Etiology of angiogenesis inhibition-related hypertension. Current Opinion in Pharmacology, 21, 7–13.
Rajendran, P., Rengarajan, T., Thangavel, J., et al. (2013). The vascular endothelium and human diseases. International Journal of Biological Sciences, 9, 1057–1069.
Dong, Z. C., Wu, M. M., Zhang, Y. L., et al. (2021). The vascular endothelial growth factor trap aflibercept induces vascular dysfunction and hypertension via attenuation of eNOS/NO signaling in mice. Acta Pharmacologica Sinica, 42, 1437–1448.
Chiu, W. C., Juang, J. M., Chang, S. N., et al. (2012). Angiotensin II regulates the LARG/RhoA/MYPT1 axis in rat vascular smooth muscle in vitro. Acta Pharmacologica Sinica, 33, 1502–1510.
Zhang, H. (2015). Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther, 9, 6075–6081.
Ding, J., Chen, X., Dai, X., et al. (2012). Simultaneous determination of apatinib and its four major metabolites in human plasma using liquid chromatography-tandem mass spectrometry and its application to a pharmacokinetic study. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 895–896, 108–115.
Cook, D. R., Rossman, K. L., & Der, C. J. (2014). Rho guanine nucleotide exchange factors: Regulators of Rho GTPase activity in development and disease. Oncogene, 33, 4021–4035.
Amin, E., Dubey, B. N., Zhang, S. C., et al. (2013). Rho-kinase: Regulation, (dys)function, and inhibition. Biological Chemistry, 394, 1399–1410.
Feng, F., Han, H., Wu, S., et al. (2021). Crosstalk between abnormal TSHR signaling activation and PTEN/PI3K in the dedifferentiation of thyroid cancer cells. Frontiers in Oncology, 11, 718578.
Chiu, W. C., Juang, J. M., Chang, S. N., et al. (2012). Differential baseline expression and angiotensin II-stimulation of leukemia-associated RhoGEF in vascular smooth muscle cells of spontaneously hypertensive rats. International Journal of Nanomedicine, 7, 5929–5939.
Dee, R. A., Mangum, K. D., Bai, X., et al. (2019). Druggable targets in the Rho pathway and their promise for therapeutic control of blood pressure. Pharmacology & Therapeutics, 193, 121–134.
Wang, Y., Zheng, X. R., Riddick, N., et al. (2009). ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circulation Research, 104, 531–540.
Shimizu, T., Fukumoto, Y., Tanaka, S., et al. (2013). Crucial role of ROCK2 in vascular smooth muscle cells for hypoxia-induced pulmonary hypertension in mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 33, 2780–2791.
Chiu, W. C., Chiang, J. Y., Juang, J. M., et al. (2020). Reduction of blood pressure elevation by losartan in spontaneously hypertensive rats through suppression of LARG expression in vascular smooth muscle cells. Journal of the Formosan Medical Association, 119, 164–172.
Zorov, D. B., Juhaszova, M., & Sollott, S. J. (2014). Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews, 94, 909–950.
Zorov, D. B., Filburn, C. R., Klotz, L. O., et al. (2000). Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. Journal of Experimental Medicine, 192, 1001–1014.
Boveris, A., Oshino, N., & Chance, B. (1972). The cellular production of hydrogen peroxide. The Biochemical Journal, 128, 617–630.
Li, L., Tan, J., Miao, Y., et al. (2015). ROS and autophagy: Interactions and molecular regulatory mechanisms. Cellular and Molecular Neurobiology, 35, 615–621.
Nair, S., & Ren, J. (2012). Autophagy and cardiovascular aging: Lesson learned from rapamycin. Cell Cycle, 11, 2092–2099.
Yu, W., Zha, W., & Ren, J. (2018). Exendin-4 and liraglutide attenuate glucose toxicity-induced cardiac injury through mTOR/ULK1-dependent autophagy. Oxidative Medicine and Cellular Longevity, 2018, 5396806.
Fan, Y. J., & Zong, W. X. (2013). The cellular decision between apoptosis and autophagy. Chinese Journal of Cancer, 32, 121–129.
Amelio, I., Melino, G., & Knight, R. A. (2011). Cell death pathology: Cross-talk with autophagy and its clinical implications. Biochemical and Biophysical Research Communications, 414, 277–281.
Lin, P. Y., Chang, C. D., Chen, Y. C., et al. (2015). RhoA/ROCK1 regulates avian reovirus S1133-induced switch from autophagy to apoptosis. BMC Veterinary Research, 11, 103.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC-81960086, NSFC-81670385 to Jing Yu) and Science and Technology Department of Gansu Province (21JR1RA150) and Education Department of Gansu Province (2021B-043), and Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (2020QN-07).
Author information
Authors and Affiliations
Contributions
Wenjuan Wang performed the experiments. Jing Yu, Qiongying Wang Caie Li, and Wenjuan Wang designed the study, and Wenjuan Wang wrote the initial draft of the manuscript. Qingjian He, Haodong Zhang, Chenchen Zhuang, and Fanxin analysed the data. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All work has been done under the guidelines of Institutional Ethics Committee.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., He, Q., Zhuang, C. et al. Apatinib Through Activating the RhoA/ROCK Signaling Pathway to Cause Dysfunction of Vascular Smooth Muscle Cells. Appl Biochem Biotechnol 194, 5367–5385 (2022). https://doi.org/10.1007/s12010-022-04020-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04020-5