Abstract
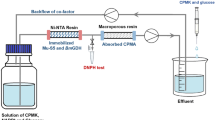
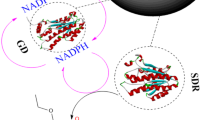
The application of immobilized enzymes in pharmaceutical and bulk chemical production has been shown to be economically viable. We demonstrate the exceptional performance of a method that immobilizes the old yellow enzyme YqjM and glucose dehydrogenase (GDH) on resin for the asymmetric hydrogenation (AH) of C = C bonds in a SpinChem reactor. When immobilized YqjM and GDH are reused 10 times, the conversion of 2-methylcyclopentenone could reach 78%. Which is because the rotor of the SpinChem reactor effectively reduces catalyst damage caused by shear force in the reaction system. When the substrate concentration is 175 mM, an 87% conversion of 2-methylcyclopentenone is obtained. The method is also observed to perform well for the AH of C = C bonds in other unsaturated carbonyl compounds with the SpinChem reactor. Thus, this method has great potential for application in the enzymatic production of chiral compounds.







Similar content being viewed by others
Data availability
All data and materials generated are included in this published article and are available.
References
Classen, T., Korpak, M., Schölzel, M., & Pietruszka, J. (2014). Stereoselective enzyme cascades: An efficient synthesis of chiral γ-butyrolactones. ACS Catalysis, 4, 1321–1331.
Lonsdale, R., & Reetz, M. T. (2015). Reduction of alpha, beta-unsaturated ketones by old yellow enzymes: Mechanistic insights from quantum mechanics/molecular mechanics calculations. Journal of the American Chemical Society, 137, 14733–14742.
Tentori, F., Bavaro, T., Brenna, E., Colombo, D., Monti, D., Semproli, R. & Ubiali, D. (2020). Immobilization of old yellow enzymes via covalent or coordination bonds. Catalysts, 10.
Szczepanska, E., Colombo, D., Tentori, F., Olejniczak, T., Brenna, E., Monti, D., & Boratynski, F. (2021). Ene-reductase transformation of massoia lactone to delta-decalactone in a continuous-flow reactor. Science and Reports, 11, 18794.
Amato, E. D., & Stewart, J. D. (2015). Applications of protein engineering to members of the old yellow enzyme family. Biotechnology Advances, 33, 624–631.
Fitzpatrick, T. B., Amrhein, N., & Macheroux, P. (2003). Characterization of YqjM, an old yellow enzyme homolog from bacillus subtilis involved in the oxidative stress response. Journal of Biological Chemistry, 278, 19891–19897.
Fitzpatrick, T. B., Auweter, S., Kitzing, K., Clausen, T., Amrhein, N., & Macheroux, P. (2004). Structural and functional impairment of an old yellow enzyme homologue upon affinity tag incorporation. Protein Expression and Purification, 36, 280–291.
Bougioukou, D. J., Kille, S., Taglieber, A., & Reetz, M. T. (2009). Directed evolution of an enantioselective enoate-reductase: Testing the utility of iterative saturation mutagenesis. Advanced Synthesis & Catalysis, 351, 3287–3305.
Clay, D., Winkler, C. K., Tasnadi, G., & Faber, K. (2014). Bioreduction and disproportionation of cyclohex-2-enone catalyzed by ene-reductase OYE-1 in ‘micro-aqueous ’ organic solvents. Biotechnology Letters, 36, 1329–1333.
Grau, M. M., van der Toorn, J. C., Otten, L. G., Macheroux, P., Taglieber, A., Zilly, F. E., Arends, I. W. â. C. â. E. and Hollmann, F. (2009). Photoenzymatic reduction of C=C double bonds. Adv. Synth. Catal., 351, 3279-3286.
Zanker, A. A., Ahmad, N., Son, T. H., Schwaminger, S. P., & Berensmeier, S. (2021). Selective ene-reductase immobilization to magnetic nanoparticles through a novel affinity tag. Biotechnology Journal, 16, e2000366.
Cui, J., Feng, Y., & Jia, S. (2018). Silica encapsulated catalase@metal-organic framework composite: A highly stable and recyclable biocatalyst. Chemical Engineering Journal, 351, 506–514.
Cui, J., & Jia, S. (2017). Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Coordination Chemistry Reviews, 352, 249–263.
Cui, J., Ren, S., Lin, T., Feng, Y., & Jia, S. (2018). Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chemical Engineering Journal, 343, 629–637.
Yoon, J., Lee, S. H., Tieves, F., Rauch, M., Hollmann, F., & Park, C. B. (2019). Light-harvesting dye–alginate hydrogel for solar-driven, sustainable biocatalysis of asymmetric hydrogenation. ACS Sustainable Chemistry & Engineering, 7, 5632–5637.
Ren, S., Li, C., Jiao, X., Jia, S., Jiang, Y., Bilal, M., & Cui, J. (2019). Recent progress in multienzymes co-immobilization and multienzyme system applications. Chemical Engineering Journal, 373, 1254–1278.
Wu, X., Hou, M., & Ge, J. (2015). Metal–organic frameworks and inorganic nanoflowers: A type of emerging inorganic crystal nanocarrier for enzyme immobilization. Catalysis Science & Technology, 5, 5077–5085.
Luan, P., Liu, Y., Li, Y., Chen, R., Huang, C., Gao, J., Hollmann, F., & Jiang, Y. (2021). Aqueous chemoenzymatic one-pot enantioselective synthesis of tertiary α-aryl cycloketones via Pd-catalyzed C-C formation and enzymatic C=C asymmetric hydrogenation. Green Chemistry, 23, 1960–1964.
Cheng, J., Zhuang, W., Tang, C., Chen, Y., Wu, J., Guo, T., & Ying, H. (2017). Efficient immobilization of AGE and NAL enzymes onto functional amino resin as recyclable and high-performance biocatalyst. Bioprocess and Biosystems Engineering, 40, 331–340.
Kahraman, M. V., Kayaman-Apohan, N., Ogan, Ae., & Güngör, A. (2006). Soybean oil based resin: A new tool for improved immobilization of α-amylase. Journal of Applied Polymer Science, 100, 4757–4761.
Contente, M. L., & Paradisi, F. (2018). Self-sustaining closed-loop multienzyme-mediated conversion of amines into alcohols in continuous reactions. Nature Catalysis, 1, 452–459.
Wang, J., Li, W., Niu, D., Singh, S., Lu, F., & Liu, X. (2017). Improved synthesis of isomaltooligosaccharides using immobilized alpha-glucosidase in organic-aqueous media. Food Sci Biotechnol, 26, 731–738.
Qian, W. Z., Ou, L., Li, C. X., Pan, J., Xu, J. H., Chen, Q., & Zheng, G. W. (2020). Evolution of glucose dehydrogenase for cofactor regeneration in bioredox processes with denaturing agents. ChemBioChem, 21, 2680–2688.
Zheng, Y. G., Yin, H. H., Yu, D. F., Chen, X., Tang, X. L., Zhang, X. J., Xue, Y. P., Wang, Y. J., & Liu, Z. Q. (2017). Recent advances in biotechnological applications of alcohol dehydrogenases. Applied Microbiology and Biotechnology, 101, 987–1001.
Wu, H., Tian, C., Song, X., Liu, C., Yang, D. and Jiang, Z. (2013). Methods for the regeneration of nicotinamide coenzymes. Green Chemistry, 15.
Zhou, J., Wu, Y., Zhang, Q., Xu, G., & Ni, Y. (2021). Co-immobilized alcohol dehydrogenase and glucose dehydrogenase with resin extraction for continuous production of chiral diaryl alcohol. Applied Biochemistry and Biotechnology, 193, 2742–2758.
Nagy, F., Gyujto, I., Tasnadi, G., Barna, B., Balogh-Weiser, D., Faber, K., Poppe, L., & Hall, M. (2020). Design and application of a bi-functional redox biocatalyst through covalent co-immobilization of ene-reductase and glucose dehydrogenase. Journal of Biotechnology, 323, 246–253.
Feng, Y., Wang, Z., Luo, Z., Chen, M., He, F., Liu, B., Goldmann, S., & Zhang, L. (2019). Further optimization of a scalable biocatalytic route to (3R)-N-Boc-3-aminoazepane with immobilized ω-transaminase. Organic Process Research & Development, 23, 355–360.
Heuschkel, I., Hanisch, S., Volke, D. C., Lofgren, E., Hoschek, A., Nikel, P. I., Karande, R., & Buhler, K. (2021). Pseudomonas taiwanensis biofilms for continuous conversion of cyclohexanone in drip flow and rotating bed reactors. Engineering in Life Sciences, 21, 258–269.
Mallin, H., Muschiol, J., Byström, E., & Bornscheuer, U. T. (2013). Efficient biocatalysis with immobilized enzymes or encapsulated whole cell microorganism by using the SpinChem reactor system. ChemCatChem, 5, 3529–3532.
Pithani, S., Karlsson, S., Emtenäs, H., & Öberg, C. T. (2019). Using SpinChem rotating bed reactor technology for immobilized enzymatic reactions: A case study. Organic Process Research & Development, 23, 1926–1931.
Szymańska, K., Odrozek, K., Zniszczoł, A., Pudło, W., & Jarzębski, A. B. (2017). A novel hierarchically structured siliceous packing to boost the performance of rotating bed enzymatic reactors. Chemical Engineering Journal, 315, 18–24.
Wachtmeister, J., Mennicken, P., Hunold, A., & Rother, D. (2016). Modularized biocatalysis: Immobilization of whole cells for preparative applications in microaqueous organic solvents. ChemCatChem, 8, 607–614.
Pesic, M., Fernández-Fueyo, E., & Hollmann, F. (2017). Characterization of the old yellow enzyme homolog from Bacillus subtilis(YqjM). ChemistrySelect, 2, 3866–3871.
Chen, R., Wei, Q., Wei, X., Liu, Y., Zhang, X., Chen, X., Yin, X., & Xie, T. (2020). Stable and efficient immobilization of bi-enzymatic NADPH cofactor recycling system under consecutive microwave irradiation. PLoS ONE, 15, e0242564.
Wachtmeister, J., Jakoblinnert, A., Kulig, J., Offermann, H., & Rother, D. (2014). Whole-cell teabag catalysis for the modularisation of synthetic enzyme cascades in micro-aqueous systems. ChemCatChem, 6, 1051–1058.
Zhang, X. J., Fan, H. H., Liu, N., Wang, X. X., Cheng, F., Liu, Z. Q., & Zheng, Y. G. (2019). A novel self-sufficient biocatalyst based on transaminase and pyridoxal 5’-phosphate covalent co-immobilization and its application in continuous biosynthesis of sitagliptin. Enyzme and Microbial Technology, 130, 109362.
Li, H., Xiao, W., Xie, P., & Zheng, L. (2018). Co-immobilization of enoate reductase with a cofactor-recycling partner enzyme. Enyzme and Microbial Technology, 109, 66–73.
Li, H., Cui, X., & Zheng, L. (2019). Functionalized poplar powder as a support material for immobilization of enoate reductase and a cofactor regeneration system. Journal of Microbiology and Biotechnology, 29, 607–616.
Gao, J., Kong, W., Zhou, L., He, Y., Ma, L., Wang, Y., Yin, L., & Jiang, Y. (2017). Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chemical Engineering Journal, 309, 70–79.
Gao, J., Wang, Y., Du, Y., Zhou, L., He, Y., Ma, L., Yin, L., Kong, W., & Jiang, Y. (2017). Construction of biocatalytic colloidosome using lipase-containing dendritic mesoporous silica nanospheres for enhanced enzyme catalysis. Chemical Engineering Journal, 317, 175–186.
Du, Y., Jia, X., Zhong, L., Jiao, Y., Zhang, Z., Wang, Z., Feng, Y., Bilal, M., Cui, J. and Jia, S. (2022). Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coordination Chemistry Reviews, 454.
Zhong, L., Jiao, X., Hu, H., Shen, X., Zhao, J., Feng, Y., Li, C., Du, Y., Cui, J., & Jia, S. (2021). Activated magnetic lipase-inorganic hybrid nanoflowers: A highly active and recyclable nanobiocatalyst for biodiesel production. Renewable Energy, 171, 825–832.
Rezaei, S., Landarani-Isfahani, A., Moghadam, M., Tangestaninejad, S., Mirkhani, V., & Mohammadpoor-Baltork, I. (2019). Development of a novel bi-enzymatic silver dendritic hierarchical nanostructure cascade catalytic system for efficient conversion of starch into gluconic acid. Chemical Engineering Journal, 356, 423–435.
Ajish, J. K., Abraham, H. M., Subramanian, M., & Kumar, K. S. A. (2021). A reusable column method using glycopolymer-functionalized resins for capture-detection of proteins and Escherichia coli. Macromolecular Bioscience, 21, e2000342.
Chen, C., Sun, W., Lv, H., Li, H., Wang, Y., & Wang, P. (2018). Spacer arm-facilitated tethering of laccase on magnetic polydopamine nanoparticles for efficient biocatalytic water treatment. Chemical Engineering Journal, 350, 949–959.
Aalbers, F. S., & Fraaije, M. W. (2017). Coupled reactions by coupled enzymes: Alcohol to lactone cascade with alcohol dehydrogenase-cyclohexanone monooxygenase fusions. Applied Microbiology and Biotechnology, 101, 7557–7565.
Li, P., Chen, Q., Wang, T. C., Vermeulen, N. A., Mehdi, B. L., Dohnalkova, A., Browning, N. D., Shen, D., Anderson, R., Gómez-Gualdrón, D. A., Cetin, F. M., Jagiello, J., Asiri, A. M., Stoddart, J. F., & Farha, O. K. (2018). Hierarchically engineered mesoporous metal-organic frameworks toward cell-free immobilized enzyme systems. Chem, 4, 1022–1034.
Lin, S., Sun, S., Wang, K., Shen, K., Ma, B., Ren, Y. and Fan, X. (2018). Bioinspired design of alcohol dehydrogenase@nano TiO2 microreactors for sustainable cycling of NAD+/NADH coenzyme. Nanomaterials (Basel), 8.
Liu, W., & Wang, P. (2007). Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnology Advances, 25, 369–384.
Kim, J.-H., Choi, G.-S., Kim, S.-B., Kim, W.-H., Lee, J.-Y., Ryu, Y.-W., & Kim, G.-J. (2004). Enhanced thermostability and tolerance of high substrate concentration of an esterase by directed evolution. Journal of Molecular Catalysis. B, Enzymatic, 27, 169–175.
Li, Y. M., Zhang, X. Y., Li, N., Xu, P., Lou, W. Y., & Zong, M. H. (2017). Biocatalytic reduction of HMF to 2,5-Bis(hydroxymethyl)furan by HMF-Tolerant Whole Cells. Chemsuschem, 10, 372–378.
Lu, C., Zhang, Z., Zhou, X., Hu, J., Ge, X., Xia, C., Zhao, J., Wang, Y., Jing, Y., Li, Y., & Zhang, Q. (2018). Effect of substrate concentration on hydrogen production by photo-fermentation in the pilot-scale baffled bioreactor. Bioresource Technology, 247, 1173–1176.
Risi, C., Zhao, F., & Castagnolo, D. (2019). Chemo-enzymatic metathesis/aromatization cascades for the synthesis of furans: Disclosing the aromatizing activity of laccase/TEMPO in oxygen-containing heterocycles. ACS Catalysis, 9, 7264–7269.
Scalacci, N., Black, G. W., Mattedi, G., Brown, N. L., Turner, N. J., & Castagnolo, D. (2017). Unveiling the biocatalytic aromatizing activity of monoamine oxidases MAO-N and 6-HDNO: Development of chemoenzymatic cascades for the synthesis of pyrroles. ACS Catalysis, 7, 1295–1300.
Acknowledgements
Thanks to Professor Frank Hollamann of the Delft University of Technology for selflessly providing the plasmid of YqjM.
Funding
This work was supported by the National Nature Science Foundation of China (Nos. 22078081, 21878068, and 21901058) and the Natural Science Foundation of Hebei Province (B2019202216).
Author information
Authors and Affiliations
Contributions
Teng Ma: Methodology, validation, formal analysis, visualization, writing (original draft), writing (review and editing).
Weixi Kong: Methodology, validation, formal analysis.
Yunting Liu: Conceptualization, formal analysis, investigation, writing (review and editing), supervision.
Hao Zhao: Writing (review and editing), supervision.
Yaping Ouyang: Validation, formal analysis.
Jing Gao: Writing (review and editing), supervision.
Liya Zhou: Writing (review and editing), supervision.
YanJun Jiang: Conceptualization, investigation, writing (review and editing), supervision.
Corresponding authors
Ethics declarations
Ethical Approval
This is an observational study. The Hebei University of Technology Research Ethics Committee has confirmed that no ethical approval is required.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
All authors agreed to publish this paper in the Journal of Applied Biochemistry and Biotechnology.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1.The enzymatic asymmetric hydrogenation of C = C bonds was investigated with a SpinChem reactor.
2.The asymmetric hydrogenation of C = C binds had an excellent performance in the SpinChem reactor.
3.The immobilized enzymes have a longer useful life in SpinChem reactor than that in other reactors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, T., Kong, W., Liu, Y. et al. Asymmetric Hydrogenation of C = C Bonds in a SpinChem Reactor by Immobilized Old Yellow Enzyme and Glucose Dehydrogenase. Appl Biochem Biotechnol 194, 4999–5016 (2022). https://doi.org/10.1007/s12010-022-03991-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03991-9