Abstract
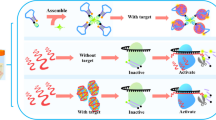
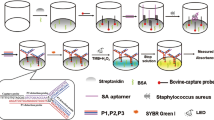
This research exhibits the design of a feasible, enzyme-free and sensitive fluorescent sensing assay for the detection of Staphylococcus aureus (S. aureus), using self-circulation of molecular beacons. With protein A on S. aureus as identifying target, the capture probe binds on the surface of S. aureus based on interaction between its aptamer section and protein A. Recognition of protein A by aptamer section in capture probe leads to allosterism of capture probe, exposing initiator section to activate the following self-circulation. After multiple circulation-based signal amplification, the method exhibits a favorable detection sensitivity and shows a promising prospect for the keratitis-related pathogenic bacteria detection. The highlights of the sensing assay are as follows: (i) capture probe is designed with aptamer section which endows the method a high selectivity; (ii) signal of bacteria is converted to nucleic acid signal after recognition of target bacteria by capture probe; and (iii) high sensitivity of method is derived from the self-circulation process. Therefore, we believe that the strategy can provide a useful platform for target bacteria detection and thus contribute to the diagnosis of infectious diseases.




Similar content being viewed by others
Data Availability
All data generated and analyzed during this study are included in this article.
References
Chen, J., Xu, Y., Yan, H., Zhu, Y., Wang, L., Zhang, Y., Lu, Y., & Xing, W. (2018). Sensitive and rapid detection of pathogenic bacteria from urine samples using multiplex recombinase polymerase amplification. Lab on a Chip, 18, 2441–2452.
Chen, J., Zhao, Y., Yao, Q. and Gao, Y. (2020) Pathological environment directed in situ peptidic supramolecular assemblies for nanomedicines. Biomedical Materials.
Gauthier, A. S., Noureddine, S., & Delbosc, B. (2019). Interstitial keratitis diagnosis and treatment. Journal Francais d’Ophtalmologie, 42, e229–e237.
Hume, E. B., Cole, N., Khan, S., Walsh, B. J., & Willcox, M. D. (2020). The role of staphopain a in Staphylococcus aureus keratitis. Experimental Eye Research, 193, 107994.
Kam, K. W., Yung, W., Li, G. K. H., Chen, L. J., & Young, A. L. (2017). Infectious keratitis and orthokeratology lens use: A systematic review. Infection, 45, 727–735.
Lakhundi, S., Siddiqui, R., & Khan, N. A. (2017). Pathogenesis of microbial keratitis. Microbial Pathogenesis, 104, 97–109.
Li, X., Ding, Y., Ling, J., Yao, W., Zha, L., Li, N., Chang, Y., Wang, Y., & Cai, J. (2019). Bacteria-targeting BSA-stabilized SiC nanoparticles as a fluorescent nanoprobe for forensic identification of saliva. Mikrochimica Acta, 186, 756.
Li, Y., Xu, F., Zhang, J., Huang, J., Shen, D., Ma, Y., Wang, X., Bian, Y., & Chen, Q. (2021). Sensitive and label-free detection of bacteria in osteomyelitis through Exo III-assisted cascade signal amplification. ACS Omega, 6, 12223–12228.
Liao, W., Lin, Q., Xie, S., He, Y., Tian, Y., & Duan, Y. (2018). A novel strategy for rapid detection of bacteria in water by the combination of three-dimensional surface-enhanced Raman scattering (3D SERS) and laser induced breakdown spectroscopy (LIBS). Analytica Chimica Acta, 1043, 64–71.
Luo, F., Li, Z., Dai, G., Lu, Y., He, P., & Wang, Q. (2020). Simultaneous detection of different bacteria by microchip electrophoresis combined with universal primer-duplex polymerase chain reaction. Journal of Chromatography A, 1615, 460734.
Russo, L., Leva Bueno, J., Bergua, J. F., Costantini, M., Giannetto, M., Puntes, V., de la Escosura-Muñiz, A., & Merkoçi, A. J. A. O. (2018). Low-cost strategy for the development of a rapid electrochemical assay for bacteria detection based on AuAg nanoshells. ACS Omega, 3, 18849–18856.
Somerville, T. F., Shankar, J., Aldwinckle, S., Sueke, H., Neal, T., Horsburgh, M. J., & Kaye, S. B. (2020). Recurrent microbial keratitis and endogenous site Staphylococcus aureus colonisation. Science and Reports, 10, 18559.
Urmann, K., Reich, P., Walter, J. G., Beckmann, D., Segal, E., & Scheper, T. (2017). Rapid and label-free detection of protein a by aptamer-tethered porous silicon nanostructures. Journal of Biotechnology, 257, 171–177.
Wei, J. (2021). Accurate and sensitive analysis of Staphylococcus aureus through CRISPR-Cas12a based recycling signal amplification cascades for early diagnosis of skin and soft tissue infections. Journal of Microbiol Methods, 183, 106167.
Wilson, S. E., & de Oliveira, R. C. (2020). Pathophysiology and treatment of diffuse lamellar keratitis. Journal of Refractive Surgery, 36, 124–130.
Wu, S., Duan, N., Shi, Z., Fang, C. and Wang, Z. J. A. C. (2014) Simultaneous aptasensor for multiplex pathogenic bacteria detection based on multicolor upconversion nanoparticles labels. 86.
Xu, L., Dai, Q., Shi, Z., Liu, X., Gao, L., Wang, Z., Zhu, X., & Li, Z. (2020). Accurate MRSA identification through dual-functional aptamer and CRISPR-Cas12a assisted rolling circle amplification. Journal of Microbiol Methods, 173, 105917.
Acknowledgements
The authors thank the financial and equipment support from Shengjing Hospital of China Medical University.
Author information
Authors and Affiliations
Contributions
D.N. designed the strategy, completed the preparation of the research, and wrote the manuscript; J.N., Z.J., Z.G., and W.T. assisted data analysis.
Corresponding author
Ethics declarations
Ethical Approval
Permission from the Institutional Animal Ethical Committee was received before making these experiments.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, N., Jiang, N., Zhao, J. et al. Sensitive and Enzyme-Free Pathogenic Bacteria Detection Through Self-Circulation of Molecular Beacon. Appl Biochem Biotechnol 194, 3668–3676 (2022). https://doi.org/10.1007/s12010-022-03948-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03948-y