Abstract
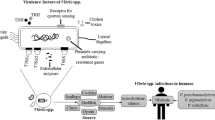
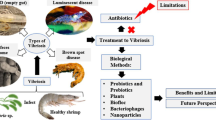
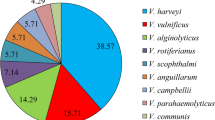
Vibriosis disease is a major threat to the aquaculture industry caused by Vibrio spp. that are often resistant to antibiotics. Alternative controlling measures such as bacteriocins could be effective due to their narrow-spectrum activity. Hence, this systematic literature review (SLR) was carried out to review the feasibility of Vibrio spp. and their vibriocins to be used as a vibriosis control measure in aquaculture. A literature search using the web of science (WOS) and SCOPUS databases resulted in 42 unique articles which were reviewed. The results showed that Vibrio spp. could be used as a probiotic to control vibriosis, but not recommended due to their opportunistic nature and pathogenesis. Vibriocin showed narrow-spectrum activity against Vibrio spp. including highly pathogenic strains such as V. alginolyticus, V. harveyi, and V. parahaemolyticus. This supported this review’s hypothesis of using vibriocin as a targeted vibriosis control measure. Vibrio cholerae was the most studied and showed the highest inhibition range, inhibiting 13 different vibrio and non-vibrio species. Various innovations were reported in the field and vibriocins can now be produced on large scales using whole-cell culture. Vibriocins were structurally diverse, large molecular weight, and relatively heat stable. These vibriocins mainly inhibited the cell wall but could have other novel mechanisms. These properties could affect the extraction process as well as applications in aquaculture, hence, should be considered in future research.

Similar content being viewed by others
Data Availability
All the data and resources used have been mentioned in the manuscript and supplementary table.
References
Pang, H., Chang, Y., Zheng, H., Tan, H., Zhou, S., Zeng, F., & Ding, Y. (2022). A live attenuated strain of HY9901ΔvscB provides protection against Vibrio alginolyticus in pearl gentian grouper (♀ Epinephelus fuscoguttatus×♂ Epinephelus lanceolatu). Aquaculture, 546, 737353.
Mohamad, A., Mursidi, F. A., Zamri-Saad, M., Amal, M. N. A., Annas, S., Monir, M. S., & Ina-Salwany, M. Y. (2022). Laboratory and field assessments of oral Vibrio vaccine indicate the potential for protection against vibriosis in cultured marine fishes. Animals, 12(2), 133.
Montánchez, I., & Kaberdina, V. R. (2020). Vibrio harveyi: a brief survey of general characteristics and recent epidemiological traits associated with climate change. Marine environmental research, 154, 104850.
Rather, I. A., Galope, R., Bajpai, V. K., Lim, J., Paek, W. K., & Park, Y. H. (2017). Diversity of marine bacteria and their bacteriocins: Applications in aquaculture. Reviews in Fisheries Science & Aquaculture, 25(4), 257–269.
Shin, J. M., Gwak, J. W., Kamarajan, P., Fenno, J. C., Rickard, A. H., & Kapila, Y. L. (2016). Biomedical applications of nisin. Journal of applied microbiology, 120(6), 1449–1465.
Wahba, A. H. (1965). Vibriocine production in the cholera and El Tor vibrios. Bulletin of the World Health Organization, 33(5), 661–664.
Hoyt, P. R., & Sizemore, R. K. (1982). Competitive dominance by a bacteriocin-producing Vibrio harveyi strain. Applied and Environmental Microbiology, 44(3), 653–658. https://doi.org/10.1128/aem.44.3.653-658.1982
McCall, J. O., & Sizemore, R. K. (1979). Description of a bacteriocinogenic plasmid in Beneckea harveyi. Applied and Environmental Microbiology, 38(5), 974–979. https://doi.org/10.1128/aem.38.5.974-979.1979
Liu, X., Wang, Z., Zhu, D., Wei, T., Gu, L., & Xu, S. (2011). Crystallization and preliminary X-ray crystallographic studies of VibE, a vibriobactin-specific 2,3-dihydroxybenzoate-AMP ligase from Vibrio cholerae. Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 67(12), 1563–1565. https://doi.org/10.1107/S1744309111039005
Bindiya, E., & Bhat, S. (2016). Marine bacteriocins: A review. J Bacteriol Mycol Open Access, 2(5), 00040.
Ha, T. Y., & Whang, H. S. (1992). Production of and sensitivity to bacteriocin, ‘vulnificin’ of Vibrio vulnificus. Journal of the Korean Society for Microbiology, 27(3), 215–230.
Burks, D. J., Norris, S., Kauffman, K. M., Joy, A., Arevalo, P., Azad, R. K., & Wildschutte, H. (2017). Environmental vibrios represent a source of antagonistic compounds that inhibit pathogenic Vibrio cholerae and Vibrio parahaemolyticus strains. MicrobiologyOpen, 6(5), e00504. https://doi.org/10.1002/mbo3.504
Balakrishnan, B., Ranishree, J. K., Thadikamala, S., & Panchatcharam, P. (2014). Purification, characterization and production optimization of a vibriocin produced by mangrove associated Vibrio parahaemolyticus. Asian Pacific Journal of Tropical Biomedicine, 4(4), 253–261. https://doi.org/10.12980/APJTB.4.2014C947
Thompson, J., Gregory, S., Plummer, S., Shields, R. J., & Rowley, A. F. (2010). An in vitro and in vivo assessment of the potential of Vibrio spp. as probiotics for the Pacific White shrimp, Litopenaeus vannamei. Journal of Applied Microbiology, 109(4), 1177–1187.
Serrano, W., Tarazona, U. I., Olaechea, R. M., & Friedrich, M. W. (2018). Draft genome sequence of a new Vibrio strain with the potential to produce bacteriocin-like inhibitory substances, isolated from the gut microflora of scallop (Argopecten purpuratus). Genome Announcements, 6(20), e00419-18. https://doi.org/10.1128/genomeA.00419-18
Selvendran, M., & Babu, M. (2013). Studies on Novel bacteriocin like inhibitory substance (BLIS) from microalgal symbiotic Vibrio spp MMB2 and its activity against aquatic bacterial pathogens. Journal of Applied Pharmaceutical Science, 3(2), 169–175.
Liu, N., Zhang, S., Zhang, W., & Li, C. (2017). Vibrio sp. 33 a potential bacterial antagonist of Vibrio splendidus pathogenic to sea cucumber (Apostichopus japonicus). Aquaculture, 470, 68–73. https://doi.org/10.1016/j.aquaculture.2016.12.028
Balcazar, J. L., Loureiro, S., Da Silva, Y. J., Pintado, J., & Planas, M. (2010). Identification and characterization of bacteria with antibacterial activities isolated from seahorses (Hippocampus guttulatus). Journal of Antibiotics, 63(5), 271–274. https://doi.org/10.1038/ja.2010.27
Farkas-Himsley, H., & Seyfried, P. L. (1962). Lethal biosynthesis of a new antibacterial principle: Vibriocin. Nature, 193(4821), 1193–1194. https://doi.org/10.1038/1931193a0
Datta, A., & Prescott, L. M. (1969). Effect of vibriocins on members of the Enterobacteriaceae. Journal of Bacteriology, 98(2), 849–850. https://doi.org/10.1128/jb.98.2.849-850.1969
Brandis, H. (1978). Chapter VI Vibriocin Typing. In T. Bergan & J. R. Norris (Eds.), Methods in Microbiology (Vol. 12, pp. 117–126). UK: Academic Press. https://doi.org/10.1016/S0580-9517(08)70360-X
Mitra, S., Balganesh, T. S., Dastidar, S. G., & Chakrabarty, A. N. (1980). Single bacteriocin typing scheme for the vibrio group of organisms. Infection and Immunity, 30(1), 74–77. https://doi.org/10.1128/iai.30.1.74-77.1980
Shehane, S. D., & Sizemore, R. K. (2002). Isolation and preliminary characterization of bacteriocins produced by Vibrio vulnificus. Journal of Applied Microbiology, 92(2), 322–328. https://doi.org/10.1046/j.1365-2672.2002.01533.x
Carraturo, A., Raieta, K., Ottaviani, D., & Russo, G. L. (2006). Inhibition of Vibrio parahaemolyticus by a bacteriocin-like inhibitory substance (BLIS) produced by Vibrio mediterranei 1. Journal of Applied Microbiology, 101(1), 234–241. https://doi.org/10.1111/j.1365-2672.2006.02909.x
Lang, D., McDonald, T. O., & Gardner, E. W. (1968). Electron microscopy of particles associated with a bacteriocinogenic Vibrio cholerae strain. Journal of Bacteriology, 95(2), 708–709. https://doi.org/10.1128/jb.95.2.708-709.1968
Smigocki, A. C., & Voll, M. J. (1986). Novel transmissible factors in a non-O1 Vibrio cholerae and a Vibrio sp. Journal of General Microbiology, 132(4), 1027–1033. https://doi.org/10.1099/00221287-132-4-1027
Sugita, H., Matsuo, N., Hirose, Y., Iwato, M., & Deguchi, Y. (1997). Vibrio sp. strain NM 10, isolated from the intestine of a Japanese coastal fish, has an inhibitory effect against Pasteurella piscicida. Applied and Environmental Microbiology, 63(12), 4986–4989. https://doi.org/10.1128/aem.63.12.4986-4989.1997
Zai, A. S., Ahmad, S., & Rasool, S. A. (2009). Bacteriocin production by indigenous marine catfish associated Vibrio spp. Pakistan Journal of Pharmaceutical Sciences, 22(2), 162–167.
Chakrabarty, A., Adhya, S., Basu, J., & Dastidar, S. G. (1970). Bacteriocin typing of Vibrio cholerae. Infection and Immunity, 1(3), 293–299.
Felsenfeld, O., & Greer, W. E. (1968). Vibriocidal activity, immune globulin producing cells and immune globulin levels in Theropithecus gelada after administration of a Vibrio cholerae antigen. Immunology, 14(3), 319–324.
Zhang, X. H., & Austin, B. (2000). Pathogenicity of Vibrio harveyi to salmonids. Journal of Fish Diseases, 23(2), 93–102. https://doi.org/10.1046/j.1365-2761.2000.00214.x
Israil, A. M., Nacescu, N., Ciufecu, C., & Cedru, C. (1987). The development and application of a bacteriocinogenotyping scheme for Vibrio cholerae non-group O-1 strains. Zentralblatt fur Bakteriologie, Mikrobiologie, und Hygiene. Series A, Medical Microbiology, Infectious Diseases, Virology, Parasitology, 264(1–2), 235–245. https://doi.org/10.1016/S0176-6724(87)80144-X
Israil, A. M., Nacescu, N., Ciufecu, C., & Stefanescu, C. (1983). Studies on bacteriocin production by NAG-strains of Vibrio cholerae as a possible epidemiologic marker. Zentralblatt fur Bakteriologie Mikrobiologie und Hygiene Abt Orig A, 255(23), 285–293.
Farkas-Himsley, H., & Cheung, R. (1976). Bacterial proteinaceous products (bacteriocins) as cytotoxic agents of neoplasia. Cancer Research, 36(10), 3561–3567.
Jayawardene, A., & Farkas-Himsley, H. (1968). Particulate nature of vibriocin: A bacteriocin from vibrio comma. Nature, 219(5149), 79–80. https://doi.org/10.1038/219079a0
Jayawardene, A., & Farkas-Himsley, H. (1970). Mode of action of vibriocin. Journal of Bacteriology, 102(2), 382–388.
Arijo, S., Brunt, J., Chabrillón, M., Díaz-Rosales, P., & Austin, B. (2008). Subcellular components of Vibrio harveyi and probiotics induce immune responses in rainbow trout Oncorhynchus mykiss (Walbaum), against V harveyi. Journal of Fish Diseases, 31(8), 579–590. https://doi.org/10.1111/j.1365-2761.2008.00932.x
Cheng, S., Zhang, W. W., Zhang, M., & Sun, L. (2010). Evaluation of the vaccine potential of a cytotoxic protease and a protective immunogen from a pathogenic Vibrio harveyi strain. Vaccine, 28(4), 1041–1047. https://doi.org/10.1016/j.vaccine.2009.10.122
Prasad, S., Morris, P. C., Hansen, R., Meaden, P. G., & Austin, B. (2005). A novel bacteriocin-like substance (BLIS) from a pathogenic strain of Vibrio harveyi. Microbiology, 151(9), 3051–3058. https://doi.org/10.1099/mic.0.28011-0
Priya, S., Santhiya, S., & Jancy, B. (2011). Vibriocin production by marine prawn associated Vibrio spp. Biomedical and Pharmacology Journal, 4(1), 227–229. https://doi.org/10.13005/bpj/286
Takeya, K., & Shimodori, S. (1969). New method for the detection of a lethal factor in vibrios. Journal of Bacteriology, 99(1), 339–340. https://doi.org/10.1128/jb.99.1.339-340.1969
Chatterjee, S. N., & Maiti, M. (1984) Vibriophages and vibriocins: Physical, chemical, and biological properties. Vol. 29. Advances in Virus Research (pp. 263–312).
Farkas-Himsley, H. (1980). Bacteriocins-are they broad-spectrum antibiotics? Journal of Antimicrobial Chemotherapy, 6(4), 424–427. https://doi.org/10.1093/jac/6.4.424
Tan, L., Gómez-Betancur, I., Guo, S., Ge, Y., Zhao, J., Chen, C., & Wang, N. (2020). Complete Genome of Vibrio neocaledonicus CGJ02-2, An active compounds producing bacterium isolated from South China Sea. Current Microbiology, 77(10), 2665–2673. https://doi.org/10.1007/s00284-020-02047-7
Somova, A. G., Ivanova, L. V., & Voronezhskaia, L. G. (1986). Importance of the vibriocinogenic factor in the ecology of Vibrio cholerae. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii, 11, 59–63.
Badalova, I. M., Mishan’kin, B. N., & Suchkov, I. I. (1987). Genetic determination of the vibriocinogenicity trait in Vibrio cholerae of the El Tor biotype. Zhurnal Mikrobiologii Epidemiologii i Immunobiologii, 5, 24–28.
Basu, S., Chakrabarty, A. N., Ganguli, M., & Dastidar, S. G. (1990). Physical demonstration of a high molecular weight bacteriocin plasmid in Vibrio cholerae by genetic transformation process. The Indian Journal of Medical Research, 91, 120–123.
Telesmanich, N. P., Vinokur, N. I., Men’shikova, E. A., & Nepomniashchaia, N. B. (2000). Bactericidal properties of hemo-cytolysin from Vibrio cholerae non O1 P-11702 strain in a panel of indicator cultures for detection of vibriocins. Zhurnal mikrobiologii, epidemiologii, i immunobiologii, 6, 74–76.
Bhaskaran, K. (1960). Recombination of characters between mutant stocks of Vibrio cholerae, strain 162. Microbiology, 23(1), 47–54.
Hernández-Cabanyero, C., Lee, C. T., Tolosa-Enguis, V., Sanjuán, E., Pajuelo, D., Reyes-López, F., & Amaro, C. (2019). Adaptation to host in Vibrio vulnificus, a zoonotic pathogen that causes septicemia in fish and humans. Environmental microbiology, 21(8), 3118–3139.
Hernández-Cabanyero, C., Sanjuán, E., Fouz, B., Pajuelo, D., Vallejos-Vidal, E., Reyes-López, F. E., & Amaro, C. (2020). The effect of the environmental temperature on the adaptation to host in the zoonotic pathogen Vibrio vulnificus. Frontiers in microbiology, 11, 489.
Phumisantiphong, U., Siripanichgon, K., Reamtong, O., & Diraphat, P. (2017). A novel bacteriocin from Enterococcus faecalis 478 exhibits a potent activity against vancomycin-resistant enterococci. PLoS One, 12(10), e0186415.
Funding
This work was supported by Talent and Publication Enhancement Research Grant (TAPE-RG) No. 55217 from University Malaysia Terengganu. We would like to thank the Centre for Research and Innovation Management (CRIM) for their assistance in gathering the resources needed in writing this review.
Author information
Authors and Affiliations
Contributions
H. I.: conceptualization, methodology, and original draft writing; A. J.: methodology and original draft writing; N. M.: review and editing of final draft and funding acquisition; L. A. R.: resources; MA: review and editing of the final draft; A. F.: validation and Formal analysis.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheikh, H., John, A., Musa, N. et al. Vibrio spp. and Their Vibriocin as a Vibriosis Control Measure in Aquaculture. Appl Biochem Biotechnol 194, 4477–4491 (2022). https://doi.org/10.1007/s12010-022-03919-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03919-3