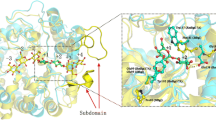
Abstract
The α-glucosidase (EC 3.2.1.20) XgtA produced by Xanthomonas campestris shows high α-glucosyl transfer activity toward alcoholic and phenolic hydroxyl groups. Ethyl vanillin-α-glucoside, a precursor-aroma compound with improved water solubility and thermal stability, can be synthesized through the transglycosylation of ethyl vanillin by XgtA. However, its low ethyl vanillin-α-glucoside yield and ability to hydrolyze ethyl vanillin-α-glucoside limits for industrial applications. Rational design and site-directed mutagenesis were employed to generate three variants of X. campestris α-glucosidase, L145I, S272T, and L145I/S272T, with improved transglycosylation activity toward EV. The highest yield is up to 52.41% by L145I/S272T, which also displayed remarkably lower hydrolysis activity toward the glycoside product EVG compared to XgtA. These results also showed that the mutation in sugar-binding subsite + 1 is more effective than subsite -1 for enhancing the ratio of transglycosylation/hydrolysis for the α-glucosidase XgtA.









Similar content being viewed by others
Data Availability
All data sources could be available to readers on request.
Code Availability
Not applicable.
References
Jung, H. J., Song, Y. S., Kim, K., Lim, C. J., & Park, E. H. (2010). Assessment of the anti-angiogenic, anti-inflammatory and antinociceptive properties of ethyl Vanillin. Archives of Pharmacal Research, 33(2), 309–316.
Desmet, T., Soetaert, W., Bojarova, P., Kren, V., Dijkhuizen, L., Eastwick-Field, V., & Schiller, A. (2012). Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chemistry - A European Journal, 18(35), 10786–10801.
Desmet, T., & Soetaert, W. (2011). Enzymatic glycosyl transfer: Mechanisms and applications. Biocatalysis and Biotransformation, 29(1), 1–18.
Kim, S. K., Kim, K. S., Ra, D. Y., & Kim, Y. H. (2003). Enzymatic synthesis of vanillin-α-glucoside and ethyl vanillin-α-glucoside. Journal of the Koresn Society of Tobacco Science, 25(2), 120–127.
Chen, L. Y., Zhou, Y. L., Lu, C. X., Ma, Z., Chen, H. C., Zhu, L. J., Lu, Y. L., & Chen, X. L. (2021). Efficient production of l-menthyl α-glucopyranoside from l-menthol via whole-cell biotransformation using recombinant Escherichia coli. Biotechnology Letters, 43(2), 1757–1764.
Bungaruang, L., Gutmann, A., & Nidetzky, B. (2013). Leloir glycosyltransferases and natural product glycosylation: Biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of redbush herbal tea. Advanced Synthesis & Catalysis, 355(14–15), 2757–2763.
Okuyama, M., Saburi, W., Mori, H., & Kimura, A. (2016). α-Glucosidases and α-1,4-glucan lyases: Structures, functions, and physiological actions. Cellular and Molecular Life Sciences, 73(14), 2727–2751.
Koshland, D. E. (1953). Stereochemistry and the mechanism of enzymatic reactions. Biological reviews, 28(4), 416–436.
Crout, D., & Vic, G. (1998). ChemInform abstract: Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. ChemInform, 2(1), 98–111.
Chen, H. C., Yang, S. S., Xu, A. J., Jiang, R. N., Tang, Z. C., Wu, J. M., Zhu, L. J., Liu, S. J., Chen, X. L., & Lu, Y. L. (2019) Insight into the glycosylation and hydrolysis kinetics of α-glucosidase in the synthesis of glycosides. Applied Microbiology and Biotechnology, 103(23-24), 9423-9432.
Banerjee, G., & Chattopadhyay, P. (2019). Vanillin biotechnology: The perspectives and future. Journal of the Science of Food and Agriculture, 99(2), 499–506.
Winter, K., Desmet, T., Devlamynck, T., Renterghem, L., Verhaeghe, T., Pelantova, H., Kren, V., & Soetaert, W. (2014). Biphasic catalysis with disaccharide phosphorylases: Chemoenzymatic synthesis of α-D-Glucosides using sucrose phosphorylase. Organic Process Research & Development, 18(6), 781–787.
Armand, S., Andrews, S. R., Charnock, S. J., & Gilbert, H. J. (2001). Influence of the aglycone region of the substrate binding cleft of Pseudomonas xylanase 10A on catalysis. Biochemistry, 40(25), 7404–7409.
Taira, T., Fujiwara, M., Dennhart, N., Hayashi, H., Onaga, S., Ohnuma, T., Letzel, T., Sakuda, S., & Fukamizo, T. (2010). Transglycosylation reaction catalyzed by a class V chitinase from cycad, Cycas revoluta: A study involving site-directed mutagenesis, HPLC, and real-time ESI-MS. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1804(4), 668–675.
Johansson, P., Brumer, H., Baumann, M. J., Kallas, A. M., Henriksson, H., Denman, S. E., Teeri, T. T., & Jones, T. A. (2004). Crystal structures of a poplar xyloglucan endotransglycosylase reveal details of transglycosylation acceptor binding. The Plant Cell, 16(4), 874–886.
Champion, E., Guerin, F., Moulis, C., Barbe, S., Thu Hoai, T., Morel, S., Descroix, K., Monsan, P., Mourey, L., Mulard, L. A., Tranier, S., Remaud, M., & Andre, I. (2012). Applying pairwise combinations of amino acid mutations for sorting out highly efficient glucosylation tools for chemo-enzymatic synthesis of bacterial oligosaccharides. Journal of the American Chemical Society, 134(45), 18677–18688.
Hui, Y. F., Drone, J., Hoffmann, L., Tran, V., Tellier, C., Rabiller, C., & Dion, M. (2005). Converting a β-glycosidase into a β-transglycosidase by directed evolution. Journal of Biological Chemistry, 280(44), 37088–37097.
Lu, Y., Yang, H. T., Hu, H. Y., Wang, Y., Rao, Z. H., & Jin, C. (2009). Mutation of Trp137 to glutamate completely removes transglycosyl activity associated with the Aspergillus fumigatus AfChiB1. Glycoconjugate Journal, 26(5), 525–534.
Arab-Jaziri, F., Bissaro, B., Tellier, C., Dion, M., Fauré, R., & O’Donohue, M. J. (2015). Enhancing the chemoenzymatic synthesis of arabinosylated xylo-oligosaccharides by GH51 α-l-arabinofuranosidase. Carbohydrate Research, 401(12), 64–72.
Teze, D., Hendrickx, J., Czjzek, M., Ropartz, D., Sanejouand, Y. H., Tran, V., Tellier, C., & Dion, M. (2014). Semi-rational approach for converting a GH1-glycosidase into a β-transglycosidase. Protein Engineering Design & Selection Peds, 27(1), 13–19.
Watanabe, R., Arimura, Y., Ishii, Y., & Kirimura, K. (2020). Crystal structure of α-glucosyl transfer enzyme XgtA from Xanthomonas campestris WU-9701. Biochemical and Biophysical Research Communications, 526(3), 580–585.
Shen, X., Saburi, W., Gai, Z., Kato, K., & Yao, M. (2015). Structural analysis of the α-glucosidase HaG provides new insights into substrate specificity and catalytic mechanism. Acta Crystallographica Section D, 71(6), 1382–1391.
Turner, P., Barber, C., & Daniels, M. (1984) Behaviour of the transposons Tn5 and Tn7 in Xanthomonas campestris pv. campestris. Molecular and General Genetics 195(1–2), 101–107.
Bissaro, B., Durand, J., Biarnes, X., Planas, A., Monsan, P., O’Donohue, M. J., & Faure, R. (2015). Molecular design of non-leloir furanose-transferring enzymes from an α-l-arabinofuranosidase: A rationale for the engineering of evolved transglycosylases. Acs Catalysis, 5(8), 4598–4611.
Champion, E., Guerin, F., Moulis, C., Barbe, S., Tran, T. H., Morel, S., Descroix, K., Monsan, P., Mourey, L., Mulard, L. A., Tranier, S., Remaud, M., & Andre, I. (2012). Applying pairwise combinations of amino acid mutations for sorting out highly efficient glucosylation tools for chemo-enzymatic synthesis of bacterial oligosaccharides. Journal of the American Chemical Society, 134(45), 18677–18688.
Osanjo, G., Dion, M., Drone, J., Solleux, C., Tran, V., Rabiller, C., & Tellier, C. (2007). Directed evolution of the α-L-fucosidase from Thermotoga maritima into an α-L-transfucosidase. Biochemistry, 46(4), 1022–1033.
Reetz, M. T., Bocola, M., Carballeira, J. D., Zha, D. X., & Vogel, A. (2005). Expanding the range of substrate acceptance of enzymes: Combinatorial active-site saturation test. Angewandte Chemie-International Edition, 44(27), 4192–4196.
Lundemo, P., Karlsson, E. N., & Adlercreutz, P. (2017). Eliminating hydrolytic activity without affecting the transglycosylation of a GH1 β-glucosidase. Applied Microbiology and Biotechnology, 101(3), 1121–1131.
Larsbrink, J., Izumi, A., Hemsworth, G. R., Davies, G. J., & Brumer, H. (2012). Structural enzymology of Cellvibrio japonicus Agd31B protein reveals α-transglucosylase activity in glycoside hydrolase family 31. The Journal of Biological Chemistry, 287(52), 43288–43299.
Funding
The financial support provided for this project was from the National Natural Science Foundation of China (Grant No. 21908196).
Author information
Authors and Affiliations
Contributions
LY Chen: Conceptualization, methodology, validation, formal analysis, investigation and writing original draft.
Y Liu: Investigation.
YY Zhou: Review and editing.
LJ Zhu and XL Chen: Conceptualization, funding acquisition, supervision, writing, review and editing.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, L., Liu, Y., Zhou, Y. et al. Improving the Transglycosylation Activity of α-Glucosidase from Xanthomonas campestris Through Semi-rational Design for the Synthesis of Ethyl Vanillin-α-Glucoside. Appl Biochem Biotechnol 194, 3082–3096 (2022). https://doi.org/10.1007/s12010-022-03908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03908-6