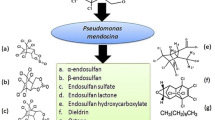
Abstract
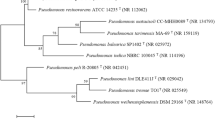
Endosulfan remains as a lipophilic insecticide that causes serious medical problems because of biological stability and toxicity also found in air, water, soil sediments, and foodstuffs. Henceforward, the present study reveals a novel bacterial species isolated from pesticide-contaminated soil for enhanced endosulfan degradation. Next, isolated bacterial species was characterized with biochemical assays and 16S rRNA sequencing technique. Subsequently, the optimal conditions for endosulfan biodegradation such as pH, concentration of endosulfan, and bacterial growth were estimated with non-sulfur medium (NSM). Sequentially, the amount of endosulfan and compound degradation were analyzed through thin-layer chromatography and gas chromatography/mass spectrometry. Overall, the obtained results revealed the endosulfan acting as primary carbon source for bacterial growth. From the GC-MS analysis, the metabolic products released during endosulfan degradation by Pseudomonas sp. MSCAS BT01 were compared with standard GC-MS spectra. The highest (98%) endosulfan degradation was obtained at pH 7.0. The complete endosulfan degradation was achieved at 14th day of incubation and the less toxic endosulfan diol produced was observed via GC-MS. To conclude, the pesticide-contaminated isolate Pseudomonas sp. MSCAS BT01 emerged as a promising bioremediation tool and effectively employed to degrade endosulfan from contaminated soils, sediments, and wastewaters in the days yet to come.










Similar content being viewed by others
References
U.S. Department of Health and Human Services. (2002). Toxicological profile for endosulfan. Agency for toxic substance and disease registry.
Beltran, F. J., García-Araya, J. F., & Acedo, B. (1994). Advanced oxidation of atrazine in water-I. Ozonation. Water Research, 28, 2153–2164.
Dyguda-Kazimierowicz, E., Roszak, S., & Sokalski, W. A. (2014). Alkaline hydrolysis of organophosphorus pesticides: The dependence of the reaction mechanism on the incoming group conformation. Journal of Physical Chemistry B., 118, 7277–7289.
Topalov, A., Abramović, B., Molnár-Gábor, D., Csanádi, J., & Arcson, O. (2001). Photocatalytic oxidation of the herbicide (4-chloro-2-methylphenoxy) acetic acid (MCPA) over TiO2. Journal of Photochemistry and Photobiology A: Chemistry., 140, 249–253.
Bahnmüller, S., Loi, C.H., Linge, K.L., Gunten, U. von, and Canonica, S. (2015) Degradation rates of benzotriazoles and benzothiazoles under UV-C irradiation and the advanced oxidation process UV/H2O2. Water Research 74, 143–154.
Semitsoglou-Tsiapou, S., Templeton, M. R., Graham, N. J. D., Hernández Leal, L., Martijn, B. J., Royce, A., et al. (2016). Low pressure UV/H2O2 treatment for the degradation of the pesticides metaldehyde, clopyralid and mecoprop - Kinetics and reaction product formation. Water Research, 91, 285–294.
Yavari, S., Sapari, N. B., Malakahmad, A., & Yavari, S. (2019). Degradation of imazapic and imazapyr herbicides in the presence of optimized oil palm empty fruit bunch and rice husk biochars in soil. Journal of Hazardous Materials, 366, 636–642.
Zwiener, C., Weil, L., & Niessner, R. (1995). UV- und UV/Ozon-Abbau von Triazinherbiziden in einer Pilotanlage - Bestimmung von Ratenkonstanten und Quantenausbeuten der UV-Photolyse. Vom Wasser, 84, 47–60.
Derylo-Marczewska, A., Blachnio, M., Marczewski, A. W., Swiatkowski, A., & Buczek, B. (2017). Adsorption of chlorophenoxy pesticides on activated carbon with gradually removed external particle layers. Chemical Engineering Journal, 308, 408–418.
Omri, A., Wali, A., & Benzina, M. (2016). Adsorption of bentazon on activated carbon prepared from Lawsonia inermis wood: Equilibrium, kinetic and thermodynamic studies. Arabian Journal of Chemistry, 9, S1729–S1739.
Wanjeri, V. W. O., Sheppard, C. J., Prinsloo, A. R. E., Ngila, J. C., & Ndungu, P. G. (2018). Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. Journal of Environmental Chemical Engineering, 6, 1333–1346.
Hengpraprom, S., Lee, C.M., and Coates, J.T. (2006) Sorption of humic acids and α-endosulfan by clay minerals. .
Sviatenko, L. K., Gorb, L., Leszczynska, D., Okovytyy, S. I., Shukla, M. K., & Leszczynski, J. (2017). In silico kinetics of alkaline hydrolysis of 1,3,5-trinitro-1,3,5-triazinane (RDX): M06-2X investigation. Environmental Science. Processes & Impacts, 19, 388–394.
Reynolds, G., Graham, N., Perry, R., & Rice, R. G. (1989). Aqueous ozonation of pesticides: A review. Ozone: Science & Engineering, 11, 339–382.
Chelme-Ayala, P., El-Din, M. G., Smith, D. W., & Adams, C. D. (2011). Oxidation kinetics of two pesticides in natural waters by ozonation and ozone combined with hydrogen peroxide. Water Research, 45, 2517–2526.
Bottrel, S., Amorim, C., Ramos, V., Romão, G., & Leao, M. (2015). Ozonation and peroxone oxidation of ethylenethiourea in water: Operational parameter optimization and by-product identification. Environmental Science and Pollution Research, 22, 903–908.
Hussain, S., Arshad, M., Saleem, M., & Zahir, Z. A. (2007). Screening of soil fungi for in vitro degradation of endosulfan. World Journal of Microbiology and Biotechnology, 23, 939–945.
Arshad, M., Hussain, S., & Saleem, M. (2008). Optimization of environmental parameters for biodegradation of alpha and beta endosulfan in soil slurry by Pseudomonas aeruginosa. Journal of Applied Microbiology, 104, 364–370.
Kafilzadeh, F., Ebrahimnezhad, M., & Tahery, Y. (2015). Isolation and identification of endosulfan-degrading bacteria and evaluation of their bioremediation in Kor River. Iran. Osong Public Health and Research Perspectives, 6, 39–46.
Kumar, K., Devi, S. S., Krishnamurthi, K., Kanade, G. S., & Chakrabarti, T. (2007). Enrichment and isolation of endosulfan degrading and detoxifying bacteria. Chemosphere, 68, 317–322.
Verma, K., Agrawal, N., Farooq, M., Misra, R. B., & Hans, R. K. (2006). Endosulfan degradation by a Rhodococcus strain isolated from earthworm gut. Ecotoxicology and Environmental Safety, 64, 377–381.
Kumar, A., Bhoot, N., Soni, I., & John, P. J. (2014). Isolation and characterization of a Bacillus subtilis strain that degrades endosulfan and endosulfan sulfate. 3. Biotech, 4, 467–475.
Singh, N. S., & Singh, D. K. (2011). Biodegradation of endosulfan and endosulfan sulfate by Achromobacter xylosoxidans strain C8B in broth medium. Biodegradation, 22, 845–857.
kumar, M. and Philip, L. (2006). Bioremediation of endosulfan contaminated soil and water-Optimization of operating conditions in laboratory scale reactors. Journal of Hazardous Materials, 136, 354–364.
Kwon, G. S., Kim, J. E., Kim, T. K., Sohn, H. Y., Koh, S. C., Shin, K. S., et al. (2002). Klebsiella pneumoniae KE-1 degrades endosulfan without formation of the toxic metabolite, endosulfan sulfate. FEMS Microbiology Letters, 215, 255–259.
Rani, R., & Kumar, V. (2017). Endosulfan degradation by selected strains of plant growth promoting rhizobacteria. Bulletin of Environmental Contamination and Toxicology, 99, 138–145.
Odukkathil, G., & Vasudevan, N. (2016). Residues of endosulfan in surface and subsurface agricultural soil and its bioremediation. Journal of Environmental Management, 165, 72–80.
Wu, S. C., Gao, J. K., & Chang, B. S. (2021). Isolation of lindane- and endosulfan-degrading bacteria and dominance analysis in the microbial communities by culture-dependent and independent methods. Microbiological Research, 251, 126817.
Casanova, A., Cabrera, S., Díaz-Ruiz, G., Hernández, S., Wacher, C., Zubillaga, M., et al. (2021). Evaluation of endosulfan degradation capacity by six pure strains isolated from a horticulture soil. Folia Microbiologica, 66, 973–981.
Ahmad, K. S. (2020). Remedial potential of bacterial and fungal strains (Bacillus subtilis, Aspergillus niger, Aspergillus flavus and Penicillium chrysogenum) against organochlorine insecticide Endosulfan. Folia Microbiologica, 65, 801–810.
Kathiravan, M. N., Karthick, R., & Muthukumar, K. (2011). Ex situ bioremediation of Cr(VI) contaminated soil by Bacillus sp.: Batch and continuous studies. Chemical Engineering Journal, 169, 107–115.
Mohanasrinivasan, V., Suganthi, V., Selvarajan, E., Subathra Devi, C., Ajith, E., Muhammed Farhan, N. P., et al. (2013). Bioremediation of endosulfan contaminated soil. Research Journal of Chemistry and Environment, 17, 93–101.
Acknowledgements
This work was supported by grants from DBT-Star Scheme and DST-FIST Scheme. The authors are grateful to the management, principal, and deans of Dr. N.G.P. Arts and Science College and Karpagam Institute of Higher Education, who provided great research facilities and infrastructures of this research.
Author information
Authors and Affiliations
Contributions
S.K.S performed the overall experiments. R.P and S.D analyzed the GC-MS results and data interpretation throughout the entire manuscript. A.R.D evaluated and designed the entire manuscript. M.N.K coordinated the entire work and wrote the entire manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Yes. All authors agreed to participate in this research.
Consent for Publication
Yes. All authors have approved the last version of the manuscript for its submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakthivel, S., Dhanapal, A.R., Palaniswamy, R. et al. Biodegradation of Endosulfan—a Chlorinated Cyclodiene Pesticide by Indigenous Pseudomonas sp. MSCAS BT01. Appl Biochem Biotechnol 194, 2747–2761 (2022). https://doi.org/10.1007/s12010-022-03869-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03869-w