Abstract
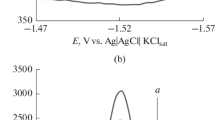
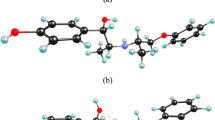
In the present paper, several computational binding analyses were performed on ethyl 3,3,5,5-tetracyano-2-hydroxy-2-methyl-4,6-diphenylcyclohexane-1-carboxylate which was newly synthesized by three-component condensation of benzaldehyde with ethyl acetoacetate and malononitrile in the presence of trichloroacetic acid, and the structure was finally proved by X-ray analysis. The visualization of molecular interaction was carried out through Hirshfeld surface analysis and ESP. The atomic charges, HOMO, LUMO, and electrostatic potential were also studied to explore the insight of the molecule deeper, and then, natural bonding orbitals (NBO) and non-linear optical properties (NLO) were calculated to reveal the interactions that happen to be between the filled and vacant orbitals. Afterwards, molecular docking studies predicted the compound binding mode fits in the minor groove of DNA and remained interacts via stable bonding as validated by molecular dynamics simulations. The binding energy estimation also affirmed domination van der Waals and electrostatic energies. Lastly, the compound was found as good drug-like molecule and had good pharmacokinetic profile with exception of toxic moieties.








Similar content being viewed by others
Availability of Data and Materials
All data and materials that were used in the article are available.
References
McGlacken, G. P., & Fairlamb, I. J. S. (2005). 2-Pyrone natural products and mimetics: Isolation, characterisation and biological activity. Natural product reports, 22(3), 369–385.
Williams, D. R., & Heidebrecht, R. W. (2003). Total synthesis of (+)-4, 5-deoxyneodolabelline. Journal of the American Chemical Society, 125(7), 1843–1850.
Pandey, G., Singh, R. P., Garg, A., & Singh, V. K. (2005). Synthesis of Mannich type products via a three-component coupling reaction. Tetrahedron Letters, 46(12), 2137–2140.
Danishefsky, S. J., Selnick, H. G., Zelle, R. E., & DeNinno, M. P. (1988). Total synthesis of zincophorin. Journal of the American Chemical Society, 110(13), 4368–4378.
Wu, J.Y.-C., Fong, W.-F., Zhang, J.-X., Leung, C.-H., Kwong, H.-L., Yang, M.-S., & Cheung, H.-Y. (2003). Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix Peucedani. European journal of pharmacology, 473(1), 9–17.
Kurbanova, M. M., Sadygova, A. Z., Gadirova, E. M., Askerov, R. K., & Maharramov, A. M. (2019). First synthesis and structure of ethyl 3, 3, 5, 5-tetracyano-2-hydroxy-2-methyl-4, 6-diphenylcyclohexane-1-carboxylate. Russian Journal of Organic Chemistry, 55(3), 381–383.
Spackman, M. A., & Jayatilaka, D. (2009). Hirshfeld surface analysis. CrystEngComm, 11(1), 19–32.
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., & Nakatsuji, H. (2016). Gaussian 16. Gaussian, Inc.
Dennington, R., Keith, T., & Millam, J. (2009). GaussView, version 5.
Stash, A. I., & Tsirelson, V. G. (2014). Developing WinXPRO: A software for determination of the multipole-model-based properties of crystals. Journal of Applied Crystallography, 47(6), 2086–2089.
Forli, W., Halliday, S., Belew, R., & Olson, A. J. (2012). AutoDock Version 4.2. Journal of Medicinal Chemistry, 55, 623–638.
Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C., & Ferrin, T. E. (2004). UCSF Chimera—a visualization system for exploratory research and analysis. Journal of computational chemistry, 25(13), 1605–1612.
Case, D. A., Belfon, K., Ben-Shalom, I. Y., Brozell, S. R., Cerutti, D. S., Cheatham, T. E. I., … Giambasu, G. (n.d.). Amber 2020, 2020. Google Scholar There is no corresponding record for this reference.
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A., & Case, D. A. (n.d.). J Comp Chem 25 (9), 1157 (2004). DOI.
Kräutler, V., Van Gunsteren, W. F., & Hünenberger, P. H. (2001). A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. Journal of computational chemistry, 22(5), 501–508.
Roe, D. R., & Cheatham, T. E., III. (2013). PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. Journal of chemical theory and computation, 9(7), 3084–3095.
Genheden, S., & Ryde, U. (2015). The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opinion on Drug Discovery, 10(5), 449–461. https://doi.org/10.1517/17460441.2015.1032936
Daina, A., Michielin, O., & Zoete, V. (2017). SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports, 7(January), 1–13. https://doi.org/10.1038/srep42717
Hodgson, J. (2001). ADMET—turning chemicals into drugs. Nature biotechnology, 19(8), 722–726.
Yang, H., Lou, C., Sun, L., Li, J., Cai, Y., Wang, Z., … Tang, Y. (2019). admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics, 35(6), 1067–1069.
Zhang, Y., & Benet, L. Z. (2001). The gut as a barrier to drug absorption. Clinical pharmacokinetics, 40(3), 159–168.
Lei, T., Sun, H., Kang, Y., Zhu, F., Liu, H., Zhou, W., … Hou, T. (2017). ADMET evaluation in drug discovery. 18. Reliable prediction of chemical-induced urinary tract toxicity by boosting machine learning approaches. Molecular pharmaceutics, 14(11), 3935–3953.
Chico, L. K., Van Eldik, L. J., & Watterson, D. M. (2009). Targeting protein kinases in central nervous system disorders. Nature reviews Drug discovery, 8(11), 892–909.
Ertl, P. (2007). In molecular drug properties: Measurement and prediction. Mannhold R. Wiley-VCH Weinheim.
Author information
Authors and Affiliations
Contributions
Malahat Kurbanova, Arzu Sadigova, and Abel Magerramov participated in the emergence of the synthesis. Rizvan Askerov contributed to X-ray analysis and Youness El Bakri, Kandasamy Saravanan, and Sajjad Ahmad took part in theoretical calculations.
Corresponding authors
Ethics declarations
Ethical Approval
All ethical norms were maintained by the authors during the article preparation process.
Consent to Participate
All authors agree to participate in the process.
Consent for Publication
All authors agree to publish the article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurbanova, M., Saravanan, K., Ahmad, S. et al. Computational Binding Analysis of Ethyl 3,3,5,5-Tetracyano-2-Hydroxy-2-Methyl-4,6-Diphenylcyclohexane-1-Carboxylate in Calf Thymus DNA. Appl Biochem Biotechnol 195, 5338–5354 (2023). https://doi.org/10.1007/s12010-022-03849-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03849-0