Abstract
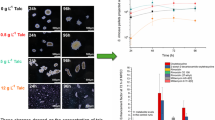
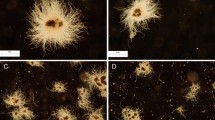
Liquid submerged fermentation is an effective strategy to achieve large-scale production of active ingredients by macrofungi, and controlling mycelium morphology is a key factor restricting the development of this technology. Mining for superior morphological regulatory factors and elucidation of their regulatory mechanisms are vital for the further development of macrofungal fermentation technology. In this study, microparticles were used to control the morphology of Paraisaria dubia (P. dubia) in submerged fermentation, and the underlying regulatory mechanisms were revealed by transcriptomic. The relative frequency of S-type pellet diameter increased significantly from 7.14 to 88.31%, and biomass increased 1.54 times when 15 g/L talc was added. Transcriptome analysis showed that the morphological regulation of filamentous fungi was a complex biological process, which involved signal transduction, mycelium polar growth, cell wall synthesis and cell division, etc. It also showed a positive impact on the basic and secondary metabolism of P. dubia. We provided a theoretical basis for controlling the mycelium morphology of P. dubia in submerged fermentation, which will promote the development of macrofungal fermentation technology.
Graphical abstract








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its additional files.
Code Availability
Not applicable.
References
Phan, C. W., Wang, J. K., Cheah, S. C., Naidu, M., David, P., & Sabaratnam, V. (2018). A review on the nucleic acid constituents in mushrooms: Nucleobases, nucleosides and nucleotides. Critical Reviews in Biotechnology, 38(5), 762–777.
Lu, H., Lou, H., Hu, J., Liu, Z., & Chen, Q. (2020). Macrofungi: A review of cultivation strategies, bioactivity, and application of mushrooms. Compr Rev Food Sci Food Saf, 19, 2333–2356.
Li, X., Liu, Q., Li, W., Li, Q., Qian, Z., Liu, X., & Dong, C. (2019). A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Critical Reviews in Biotechnology, 39(2), 181–191.
Baby, S., Johnson, A. J., & Govindan, B. (2015). Secondary metabolites from Ganoderma. Phytochemistry, 114, 66–101.
Papinutti, L. (2010). Effects of nutrients, pH and water potential on exopolysaccharides production by a fungal strain belonging to Ganoderma lucidum complex. Bioresource Technology, 101(6), 1941–1946.
Papagianni, M. (2004). Fungal morphology and metabolite production in submerged mycelial processes. Biotechnology Advances, 22(3), 189–259.
Zhang, J., & Zhang, J. (2016). The filamentous fungal pellet and forces driving its formation. Critical Reviews in Biotechnology, 36(6), 1066–1077.
Karahalil, E., Coban, H. B., & Turhan, I. (2018). A current approach to the control of filamentous fungal growth in media: Microparticle enhanced cultivation technique. Critical Reviews in Biotechnology, 39(2), 192–201.
Krull, R., Wucherpfennig, T., Esfandabadi, M. E., Walisko, R., Melzer, G., Hempel, D. C., Kampen, I., Kwade, A., & Wittmann, C. (2013). Characterization and control of fungal morphology for improved production performance in biotechnology. Journal of Biotechnology, 163(2), 112–123.
Kurakake, M., Hirotsu, S., Shibata, M., Takenaka, Y., Kamioka, T., & Sakamoto, T. (2017). Effects of nonionic surfactants on pellet formation and the production of beta-fructofuranosidases from Aspergillus oryzae KB. Food Chemistry, 224, 139–143.
Wei, L., Liu, Y., Frear, C., & Chen, S. (2008). Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material - dairy manure - using a pelletized filamentous fungus Rhizopus oryzae ATCC 20344. Bioresource Technology, 99(13), 5859–5866.
López, J. L. C., Pérez, J. A. S., Sevilla, J. M. F., Porcel, E. M. R., & Chisti, Y. (2005). Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus. Journal of Biotechnology, 116(1), 61–77.
Tao, T.L., Cui, F.J., Chen, X.X., Sun, W.J., Huang, D.M., Zhang, J., Yang, Y., Wu, D. & Liu, W.M. (2018) Improved mycelia and polysaccharide production of Grifola frondosa by controlling morphology with microparticle Talc. Microb Cell Fact, 17(1).
Saberi, A., Jalili, H., Nikfarjam, A., Koohsorkhi, J., Jarmoshti, J., & Bizukojc, M. (2020). Monitoring of Aspergillus terreus morphology for the lovastatin production in submerge culture by impedimetry. Bioprocess Biosyst Eng, 159, 107615.
Gonciarz, J., & Bizukojc, M. (2014). Adding talc microparticles to Aspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Engineering in Life Sciences, 14(2), 190–200.
Wang, Y., Li, Z. L., Suo, F. Y., & Sun, D. P. (2019). Study of mycelial polysaccharide from Paraisaria dubia of Ophiocordyceps gracilis asexual. China journal of Chinese materia medica, 44, 1704–1709.
Cui, J. D. (2015). Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Critical Reviews in Biotechnology, 35, 475–484.
Meng, Q., Lu, C., Gao, H., Chen, G., Wu, L., Wu, J., Li, S. & He, B. F. (2021) Efficient biosynthesis of exopolysaccharide from Jerusalem artichoke using a novel strain of Bacillus velezensis LT-2. Bioresour Technol, 320(Pt A), 124346.
Conesa, A., Götz, S., García-Gómez, J. M., Terol, J., Talón, M., & Robles, M. (2005). Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics, 21(18), 3674–3676.
Ogata, H., Goto, S., Sato, K., Fujibuchi, W., Bono, H., & Kanehisa, M. (1999). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research, 27(1), 29–34.
Wucherpfennig, T., Kiep, K. A., Driouch, H., Wittmann, C., & Krull, R. (2010). Morphology and rheology in filamentous cultivations. Advances in Applied Microbiology, 72, 89–136.
Veiter, L., Rajamanickam, V., & Herwig, C. (2018). The filamentous fungal pellet-relationship between morphology and productivity. Applied Microbiology and Biotechnology, 102(7), 2997–3006.
Driouch, H., Sommer, B., & Wittmann, C. (2010). Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnology and Bioengineering, 105, 1058–1068.
Coban, H., Demirci, A., & Turhan, I. (2015). Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess and Biosystems Engineering, 38(6), 1075–1080.
Erick Eligio, A. P., Gabriela, G. C., Gloria, S. C., Dimitris, G., & Luis, S. G. (2019). A novel two-component system, encoded by the sco5282/sco5283 genes, affects Streptomyces coelicolor morphology in liquid culture. Frontiers in Microbiology, 10, 1568.
Zacchetti, B., Smits, P., & Claessen, D. (2018). Dynamics of pellet fragmentation and aggregation in liquid-grown cultures of Streptomyces lividans. Frontiers in Microbiology, 9, 943.
Zacchetti, B., Willemse, J., Recter, B., van Dissel, D., van Wezel, G. P., Wösten, H. A. B., & Claessen, D. (2016). Aggregation of germlings is a major contributing factor towards mycelial heterogeneity of Streptomyces. Science and Reports, 6, 27045.
Chen, X., Zhou, J., Ding, Q., Luo, Q., & Liu, L. (2019). Morphology engineering of Aspergillus oryzae for l-malate production. Biotechnology and Bioengineering, 116(10), 2662–2673.
Domingo, M. S., & José, R. H. (2017). Functional analysis of the MAPK pathways in fungi. Revista Iberoamericana de Micología, 34(4), 192–202.
Chen, H., Zhou, X., Ren, B., & Cheng, L. (2020). The regulation of hyphae growth in Candida albicans. Virulence, 11(1), 337–348.
Chow, J., Dionne, H. M., Prabhakar, A., Mehrotra, A., Somboonthum, J., Gonzalez, B., Edgerton, M. & Cullen, P. J. (2019) Aggregate filamentous growth responses in yeast. mSphere, 4 (2), e00702–18..
Wang, J., Wen, X., Yang, B., Liu, D., Li, X., & Geng, F. (2020). De novo transcriptome and proteome analysis of Dictyophora indusiata fruiting bodies provides insights into the changes during morphological development. International Journal of Biological Macromolecules, 146, 875–886.
Liu, H., Zheng, Z., Wang, P., Gong, G., Wang, L., & Zhao, G. (2013). Morphological changes induced by class III chitin synthase gene silencing could enhance penicillin production of Penicillium chrysogenum. Applied Microbiology and Biotechnology, 97(8), 3363–3372.
Müller, C., McIntyre, M., Hansen, K., & Nielsen, J. (2002). Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Applied and Environment Microbiology, 68(4), 1827–1836.
Yu, P., Chang, Y., Liou, R., Lee, T., & Tzean, S. (2016). pks63787, a polyketide synthase gene responsible for the biosynthesis of benzenoids in the medicinal mushroom Antrodia cinnamomea. Journal of Natural Products, 79(6), 1485–1491.
Suo, F. Y., Huang, L. D., & Yu, H. (2014). Identification and antibacterial effect research of a Tolypocladium strain isolated from sclerotium of Ophiocordyceps gracilis in Xinjiang. China Journal of Chinese Materia Medica, 39, 965.
Funding
This work was supported by the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project TSBICIP-PTJS-003-04 and Natural Science Foundation of Jiangsu Province, China (Grant No. BK20200732) .
Author information
Authors and Affiliations
Contributions
TLL and WY conceived and designed the experiments. TLL performed the experiments. WY, DYH, YL, LMZ, MXY, CZL, ZYD, and HSJ analyzed the data. TLL and WY wrote the manuscript with contributions from all authors. GDS and LXJ supervised the whole research work and revised the manuscript. All authors read and comment on the manuscript before submission.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
All authors agreed to publish this article.
Human and Animal Participants
No such procedures were performed in studies.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tong, LL., Wang, Y., Du, YH. et al. Transcriptomic Analysis of Morphology Regulatory Mechanisms of Microparticles to Paraisaria dubia in Submerged Fermentation. Appl Biochem Biotechnol 194, 4333–4347 (2022). https://doi.org/10.1007/s12010-022-03820-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03820-z