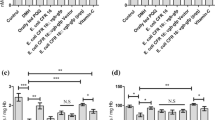
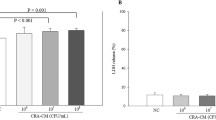
Abstract
Contaminated rice is a major source of food poisoning in human communities where our earlier study showed Stenotrophomonas maltophilia, a Gram-negative bacillus, has been a major contaminant of the stored rice. In the present study, mono- and di-unsaturated fatty acids (UFAs) such as 18:1 ω 7 c, 16:1 ω 6 c, 16:1 ω 7 c, and 18:2 ω 6,9 c long-chain fatty acids have been found as the chief constituents of S. maltophilia boiled cell lysate. Throughout the study, both acute and chronic exposure of the cell lysate showed a decrease in the locomotor activity and a time-dependent increase of the depression (p < 0.001–0.0001, two-way ANOVA), supported by bioamine (dopamine, noradrenaline, adrenaline, serotonin, and GABA) depletion in rodents’ brain possibly due to UFA-amino acid decarboxylase interaction favoring bioamine depletion as revealed by our study. Furthermore, the UFA-rich cell lysate revealed dose-dependent inhibition of murine brain microglial cell viability in vitro with concomitant increase of reactive oxygen species (ROS) inside the cell. Destruction of neuroprotective and neurotrophin releasing microglial cells, augmentation of brain ROS, and inflaming brain tissue resulting in infiltration of polymorphonuclear leucocytes also suggest to cause neurotoxicity by UFA derived from Stenotrophomonas maltophilia.
Graphical abstract










Similar content being viewed by others
Availability of Data and Materials
All data and materials support published claims and comply with field standards.
References
Kumar, D., & Kalita, P. (2017). Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods., 6, 1–22.
Abedin, M. Z., Rahman, M. Z., Mia, M. I. A., & Rahman, K. M. M. (2012). In-store losses of rice and ways of reducing such losses at farmers’ level: An assessment in selected regions of Bangladesh. J. Bangladesh. Agri. Univ., 10, 133–144.
Baumgardner, D. J. (2012). Soil-related bacterial and fungal infections. Journal of the American Board of Family Medicine, 25, 734–744.
Leyva Salas, M., Mounier, J., Valence, F., Coton, M., Thierry, A., & Coton, E. (2017). Antifungal microbial agents for food biopreservation—A review. Microorganisms., 5, 1–35.
Food Safety-World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/food-safety. Accessed March 15, 2019.
Chattopadhyay, M., Das, D., Das, D., Gupta, M., & Bagchi, G. (2018). Assessment of hematological and biochemical effects of acute and chronic administration of the Stenotrophomonas maltophilia lysates in mice. Journal of Microbiology, Biotechnology and Food Sciences, 8, 936–939.
Lin, C. M., Fernando, S. Y., & Wei, C. (1996). Occurrence of Listeria monocytogenes, Salmonella spp., Escherichia coli and E. coli O157:H7 in vegetable salads. Food Control, 7, 135–140.
Zhu, B., Liu, H., Tian, W. X., Fan, X. Y., Li, B., Zhou, X. P., Jin, G., & Xie, G. (2018). Genome sequence of Stenotrophomonas maltophilia RR-10, isolated as an endophyte from rice root. Journal of Bacteriology, 194, 1280–1291.
Denton, M., & Kerr, K. G. (1998). Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clinical Microbiology Reviews, 11, 57–80.
Steinmann, J., Mamat, U., Abda, E., Kirchhoff, L., Streit, W., Schaible, U., Niemann, S., & Kohl, T. (2018). Analysis of phylogenetic variation of Stenotrophomonas maltophilia reveals human-specific branches. Frontiers in Microbiology, 9, 1–10.
O’Leary, W. M. (1962). The fatty acids of bacteria. Bacteriological Reviews, 26, 421–447.
Sekora, N. S., Lawrence, K. S., Agudelo, P., Santen, E., & McInroy, J. A. (2009). Using FAME analysis to compare, differentiate, and identify multiple nematode species. Journal of Nematology, 41, 163–173.
Jayasree, T., Naveen, A., Chandrasekhar, N., Sunil, M., Kishan, P., & Rao, N. (2012). Evaluation of muscle relaxant activity of aqueous extract of Sapindustri foliatus (pericarp) in Swiss albino mice. J. Chem. Pharm. Res, 4, 1960–1964.
Can, A., Dao, D. T., Arad, M., Terrillion, C. E., Piantadosi, S. C., & Gould, T. D. (2012). The mouse forced swim test. Journal of Visualized Experiments, 59, 1–5.
Atack, C. V. (1973). The determination of dopamine by a modification of the dihydroxyindolefluorimetric assay. British Journal of Pharmacology, 48, 699–714.
Carmona, E., Gomes, C., & Trolin, G. (1980). Purification of GABA on small columns of Dowex 50 w; combination with a method for separation of biogenic amines. Acta Pharmacologica et Toxicologica, 46, 235–240.
Drury, R. A., Wallington, E. A., & Cameron, R. (1967). Carlton’s histological technique (4th ed.). Oxford University Pres.
Alturkistani, H. A., Tashkandi, F. M., & Mohammedsaleh, Z. M. (2016). Histological stains: A literature review and case study. Glob. J. Health Sci., 8, 72–79.
Grenham, S., Clarke, G., Cryan, J. F., & Dinan, T. G. (2011). Brain–gut–microbe communication in health and disease. Frontiers in Physiology, 2, 94–105.
Barret, A.D.T. and Stanberry, L. (2009). Vaccine adjuvants. Vaccines for biodefense and emerging and neglected diseases. 1st ed.; Academic Press, Cambridge, Massachusetts.
Ryan, J. (2004). Endotoxins and cell culture. Corning Life Sci. Tech. Bull. 1–8.
Figuera-Losada, M., Rojas, C., & Slusher, B. S. (2014). Inhibition of microglia activation as a phenotypic assay in early drug discovery. Journal of Biomolecular Screening, 19, 17–31.
Kelly, M. A., Rubinstein, M., Phillips, T. J., Lessov, C. N., Burkhart-Kasch, S., Zhang, G., Bunzow, J. R., Fang, Y., Gerhardt, G. A., Grandy, D. K., & Low, M. J. (1998). Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. Journal of Neuroscience, 18, 3470–3479.
Krishnan, V., & Nestler, E. J. (2011). Models of depression: Molecular perspectives Curr. Top. Behav. Neurosci., 7, 121–147.
Jacobsen, J. P., Medvedev, I. O., & Caron, M. G. (2012). The 5-HT deficiency theory of depression: Perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences, 367, 2444–2459.
Luscher, B., Shen, Q., & Sahir, N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Molecular Psychiatry, 16, 383–406.
Fogaca, M. V., & Duman, R. S. (2019). Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Frontiers in Cellular Neuroscience, 13, 87. https://doi.org/10.3389/fncel.2019.00087
Cryan, J. F., & Slattery, D. A. (2010). GABAB receptors and depression. Current status. Adv. Pharmacol., 58, 427–451.
Kobayashi, K. (2001). Role of catecholamine signaling in brain and nervous system functions: New insights from mouse molecular genetic study. The Journal of Investigative Dermatology, 6, 115–121.
Nicolson, G. L. (2008). Chronic bacterial and viral infections in neurodegenerative and neurobehavioural disease. Laboratoriums Medizin, 39, 1–5.
Precht, W., & Yoshida, M. (1971). Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Research, 32, 229–233.
Chen, Y., Garcia, G. E., Huang, W., & Constantini, S. (2014). The involvement of secondary neuronal damage in the development of neuropsychiatric disorders following brain insults. Frontiers in Neuroscience, 5, 22–26.
Menniti, F. S., & Diliberto, J. E., Jr. (1989). Newly synthesized dopamine as the precursor for norepinephrine synthesis in bovine adrenomedullary chromaffin cells. Journal of Neurochemistry, 53, 890–897.
Hallenbeck, J. M., Dutka, A. J., Tanishima, T., Kochanek, P. M., Kumaroo, K. K., Thompson, C. B., Obrenovitch, T. P., & Contreras, T. J. (1986). Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke, 17, 246–253.
Liu, Y. W., Li, S., & Dai, S. S. (2018). Neutrophils in traumatic brain injury (TBI): Friend or foe? Journal of Neuroinflammation, 15, 1–18.
Acknowledgements
We are thankful to the NCCS (National Centre for Cell Science), Pune, India, for helping us in finding the free fatty acid present in bacteria and Bose Institute, Kolkata, India, to carry out the analytical work in their Central Instrumentation Facility. We are also thankful to the CPCSEA approved animal house of Dr. B.C. Roy College of Pharmacy & Allied Health Sciences, Durgapur, WB, India, for providing us the facility for conducting the animal studies in the research.
Author information
Authors and Affiliations
Contributions
Experimental investigations (animal studies, bioamine determination)—Moitreyee Chattopadhyay, Dibyajyoti Das; work plan and conceptualization, molecular docking analyses, bioamine determination—Souvik Basak; data interpretation, original writing—Moitreyee Chattopadhyay, Souvik Basak; cell culture assays, data interpretation—Atish Barua; planning and guiding—Malaya Gupta, Gautam Kumar Bagchi; statistical analyses—Tanushree Karmakar.
Corresponding authors
Ethics declarations
Ethics Approval
The experimentation on laboratory animals was conducted according to the regulations of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) in India and the protocol was approved by Institutional Animal Ethics Committee of Dr. B.C. Roy College of Pharmacy and Allied Sciences, Durgapur, India (Approval Number: BCRCP/IAEC/1/2014).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chattopadhyay, M., Basak, S., Barua, A. et al. Neuropharmacological Alterations by a Rice Contaminant Stenotrophomonas maltophilia: a Detailed Bio-molecular and Mechanistic Landscape. Appl Biochem Biotechnol 194, 1955–1980 (2022). https://doi.org/10.1007/s12010-022-03810-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03810-1