Abstract
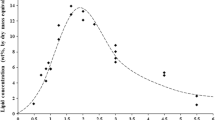
Microalgae have the potential to emerge as renewable feedstocks to replace fossil resources in producing biofuels and chemicals. Levulinic acid is one of the most promising substances which may serve as chemical building blocks. This work investigated the use of Spirulina platensis residue (solid residue after lipids extraction) to produce LA via acid hydrolysis reaction. In this study, Spirulina platensis residue was set to have a solid–liquid ratio of 5% (w/v). The effect of process parameters on the Spirulina platensis residue to levulinic acid hydrolysis reaction was observed at temperatures ranging from 140 to 180 °C under four acid concentrations, i.e., 0.25, 0.5, 0.8, and 1 M. A simplified kinetic model was also developed to describe the behavior of Spirulina platensis residue conversion to levulinic acid, based on the pseudo-homogeneous–irreversible–1st order reaction. The results showed that the proposed model could capture the experimental data well. The reaction network also considered involvement of intermediate products namely glucose and 5-hydroxymethylfurfural. The results showed that Spirulina platensis residue, with acid catalysts, can be used to produce levulinic acid, and the kinetic model can provide useful information for understanding the Spirulina platensis residue to levulinic acid hydrolysis reaction.







Similar content being viewed by others
Data Availability
All data and materials generated are included in this published article and are available upon reasonable request.
Code Availability
Glu: Glucose.
5-HMF: 5-hydrovymethylfurfural.
LA: Levulinic acid.
Ri: Reaction rate of i [mol/L/min].
ki: Coefficient of reaction kinetic of i [1/min].
Ci: Concentration of I [mol/L].
Ai: Arrhenius constant of i.
Ei: Activation energy of I [kJ/mol].
R: Ideal gas constant [J/K/mol].
T: Temperature [K].
SSE: Sum of squared error.
References
Galletti, A.M.R., Antonetti, C., Licursi, D., Mussi, L., Balestri, E., and Lardicci, C. (2019) AIDIC, 74, 103-108
Antonetti, C., Licurci, D., Fulignati, S., Valentini, G., and Galetti, A.M.R. (2016) Catalyst, 6, 196
Deviram, G., Mathimani, T., Anto, S., Ahamed, T.S., Ananth, D.A., & Pugazhendhi, A., (2020) J. Cleaner Prod., 253, 119770
Listyaningrum, N. B., Azis, M.M., Sarto, Rosdi, A. N., and Harun, M. R. (2021) AJChE 21(1), 11–18.
Aharonovich, E.B., Zandany, A., Saady, A., Tahan, Y.K., Yehoshua, Y., and Gedanken, A. (2020) Bioresour. Technol. Rep. 11, 100514.
Jeong, G.T. and Kim, S.K. (2021) Fuel 283, 118907.
Rackemann, D.W. (2014), PhD Disertasi, Queensland University of Technology.
Signoretto, M., Taghavi, S., Ghedini, E., and Menegazzo, F. (2019) Molecules 24, 1-20
Nautiyal, P., Subramanian, K.A., and Dastidar, M.G. (2014) Fuel 135, 228-234
Jamilatun, S., Budhijanto, Rochmadi, Yuliestyan, A., Aziz, M., Hayashi, J.I., and Budiman, A. (2020) IJTech 11(3), 522–531.
Jamilatun, S., Budhijanto, Rochmadi, Yuliestyan, A., Hadiyanto, H. and Budiman, A. (2019) IJRED 8 (2), 133–140.
Aikawa, S., Hsin Ho, S., Nakanishi, A., Shu Chang, J., Hasunuma, T., and Kondo, A., (2015) Biotechnology Journal. 10, 886-898
Izumi, Y., Aikawa, S., Matsuda, F., and Hasunima, T. (2013) Journal of Chromatography. B. 930, 90-97
Vonshak A. (1997) Spirulina: growth, physiology & biochemistry. In: Spirulina platensis (Arthrospira): physiology, cell biology and biotechnology, Taylor and Francis, London,
Vernes, L., Abert-Vian, M., El Maataoui, M., Tao, Y., Bornard, I. and Chemat, F. (2019) Ultrason. Sonochem.
Halim, R., Harun, R., Danquah M. K and Webley, P.A. (2012) Applied Energy 91, 116-121
Khan, M. I., Shin J. H., and Kim, J. D. (2018) Microb. Cell Fact., 17–36.
Girisuta, B., Janssen, L. P. B. M., and Heeres, H.J. (2007) Industrial and Engineering Chemistry Research 46, 1696-1708
Chun, C., Xiaojian, M., and Peilin, C. (2009) Chinese. Journal of Chemical Engineering 17(5), 835-839
L. Kupiainen, J. Ahola, J. Tanskanen (2011) Chemical Engineering Research and Design 89 (2011) 2706-2713
Ahlkvist, J. (2014), VMC-KBC Umeå , Umeå, Sweden, ISBN: 978-91-7459-798-1
Morone, A., Apte, M., and Pandey, R.A. (2015) Renewable and Sustainable Energy Rev., 51, 548-565
Thapa, I., Mullen, B., Saleem, A., Leibig, C., Baker, R.T., and Giorgi, J.B. (2017) Applied Catalysis, A, 539, 70-79
Fang, Q. and Hanna, M.A. (2002) Bioresource. Technology., 81, 187–192
Lopes, E.S., Dominicesa, K.M.C., Lopes, M.S., and Tovar, L.P., Filhoa, R.M. (2017) Chem. Eng. Transact., AIDIC, 57
Kang, M., Kim, S.W., Kim, J.W., Kim, T.H., & Kim, J.S. (2013) Renewable Energy, 54, 173-180
Lee, S.B., Kim, S.K., Hong, Y.K., and Jeong, G.T. (2016) Algal Research 13, 303-310
Jeong, G.T., Ra, C.H., Hong, Y.K., Kim, J.K., Kong, I.S., Kim, S.K., and Park, D.H. (2015) Bioprocess and Biosystems. Engineering 38, 207–218
Kim, D.H., Lee, S.B., Kim, S.K., Park, D.H., and Jeong, G.T. (2016) Bioenerg.Res.
Saeman, J. F. (1945) Industrial and Engineering Chemistry, 37, 43-53
Girisuta B., Dussan K., Haverty D., Leahy J., and Hayes M. (2013) Chemical Engineering Journal 217, 61-70
Zheng, X., Zhi, Z., Gu, X., Li, X., Zhang, R., and Lu, X. (2017) Fuel 187, 261-268
Fogler, H. S. (2016). Element Of Chemical Reaction Engineering (5th ed.). Prentice Hall.
Toif, M.E., Hidayat, M., Rochmadi, and Budiman, A. (2020) AIP Conf. Proc. 2296, 020064, 1-6
Toif, M.E., Hidayat, M., Rochmadi, and Budiman, A. (2021) BCREC 16 (4), 904–915.
Yang G, Pidko EA, and Hensen EJM (2012) Journal of Catalysis 295, 122–32
Hu L, Lin L, Wu Z, Zhou S, and Liu S. (2017) Renewable and Sustainable Energy Reviews 74, 230–57
Latham, K. G., Ferguson, A., and Donne, S. (2018) SN Applied Science, 2019, 1:54
De Souza, R. L., Yu, H., Rataboul, F., and Essayem, N., (2012) Challenges 3, 212-232
Yu, I. K. M., and Tsang, D. C.W. (2017) Biosource Technology 238, 716-732
Cao, L., Yu, I.K.M., Cho, D.W., Wang, D., Tsang, D.C.W., Zhang, S., Ding, S., Wang, L., and Ok, Y.S. (2019) Bioresource Technology 273, 251-259
Acknowledgements
The authors are grateful to Ministry of Research and Technology/National Research and Innovation Agency (RISTEK-BRIN), Republic of Indonesia, for the research funding.
Funding
Kementerian Riset,Teknologi dan Pendidikan Tinggi
Author information
Authors and Affiliations
Contributions
Arief Budiman conceived the main idea of this experiment. Rochmadi designed the detailed experiment. Material preparation, data collection and analysis were performed by Retno Ringgani. The mathematical calculation and modelling were performed by Retno Ringgani and Muhammad Mufti Azis. The first draft of the manuscript was written by Retno Ringgani and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent to Participate
The authors have consented to the submission of this report to the journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ringgani, R., Azis, M.M., Rochmadi et al. Kinetic Study of Levulinic Acid from Spirulina platensis Residue. Appl Biochem Biotechnol 194, 2684–2699 (2022). https://doi.org/10.1007/s12010-022-03806-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03806-x