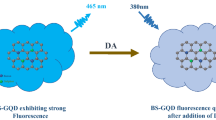
Abstract
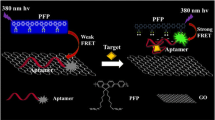
Dopamine (DA) is a catecholamine neurotransmitter playing an important role in different biological functions including central nervous, renal, cardiovascular, and hormonal systems. The sensitive and selective detection of this neurotransmitter plays a key role in the early diagnosis of various diseases related to abnormal levels of dopamine. Therefore, it is of great importance to explore rapid, simple, and accurate methods for detection of dopamine with high sensitivity and specificity. We propose in this work a fluorescent aptasensor based on graphene oxide (GO) as a quencher, for the rapid determination of dopamine. The principle of this aptasensor is based on fluorescence resonance energy transfer (FRET), where GO was used as energy donor, and a carboxy fluorescein (FAM)-labeled aptamer as acceptor. In the absence of DA, FAM-aptamer was adsorbed on the surface of GO through π-π stacking interactions between nucleotide bases and the carbon network, leading to a weak FRET and a quenching of the FAM fluorescence. However, by adding the target, the aptamer undergoes a conformational change to bind to DA with high affinity, resulting in a fluorescence recovery. Under the optimal experimental conditions, the fluorescence recovery was linearly proportional to the concentration of DA in the range of 3–1680 nM, with a limit of detection of 0.031 nM and a limit of quantification of 0.1 nM. Moreover, the developed assay exhibited minor response in the presence of various interferents and it revealed a satisfactory applicability in human serum samples.







Similar content being viewed by others
Data Availability
Not applicable
Code Availability
Not applicable
References
Shang, N. G., Papakonstantinou, P., McMullan, M., Chu, M., Stamboulis, A., Potenza, A., Dhesi, S. S., Marchetto, H. J. A. F. M. (2008) Catalyst‐free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. 18, 3506-3514.
Bucolo, C., Leggio, G. M., Drago, F., Salomone, S. J. P., therapeutics (2019) Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. 203, 107392.
Fischer, N. M., Hinkle, J. T., Perepezko, K., Bakker, C. C., Morris, M., Broen, M. P., Butala, A., Dawson, T. M., Leentjens, A. F., Mari, Z. (2021). Brainstem pathologies correlate with depression and psychosis in parkinson's disease.
Dias, A. S. A., Pinto, J. C. A., Magalhães, M., Mendes, V. M., Manadas, B. J. J., Analysis, B. (2020). Analytical methods to monitor dopamine metabolism in plasma: Moving forward with improved diagnosis and treatment of neurological disorders. 187, 113323.
Pérez-Fernández, V., Harman, D. G., Morley, J. W., Cameron, M. A. J. A. (2017). Optimized method to quantify dopamine turnover in the mammalian retina. 89, 12276-12283.
Sahoo, H. J. J. o. P., Reviews, P. C. P. (2011). Förster resonance energy transfer–A spectroscopic nanoruler: Principle and application, 12, 20-30.
Medintz, I. L., & Hildebrandt, N. (2013). FRET-Förster resonance energy transfer: From theory to applications. John Wiley & Sons.
Pehlivan, Z. S., Torabfam, M., Kurt, H., Ow-Yang, C., Hildebrandt, N., & Yüce, M. J. M. A. (2019). Aptamer and nanomaterial based FRET biosensors: A review on recent advances (2014–2019). Mikrochim Acta, 186, 1–22.
Shi, J., Tian, F., Lyu, J., Yang, M. (2015). Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. 3, 6989-7005.
Seok, Y., Byun, J. Y., Shim, W. B., & Kim, M. G. J. A. C. A. (2015). A structure-switchable aptasensor for aflatoxin B1 detection based on assembly of an aptamer/split DNAzyme. Anal Chim Acta, 886, 182–187.
Mahmoudpour, M., Ding, S., Lyu, Z., Ebrahimi, G., Du, D., Dolatabadi, J. E. N., Torbati, M., Lin, Y. J. N. T. (2021) Aptamer functionalized nanomaterials for biomedical applications. Recent Advances and New Horizons. 39, 101177.
Vo-Dinh, T., (2003) Novel fluorescent molecular beacon DNA probes for biomolecular recognition, CRC Press, pp. 1533-1556.
Song, S., Liang, Z., Zhang, J., Wang, L., Li, G., & Fan, C. J. A. C. (2009). Gold-nanoparticle-based multicolor nanobeacons for sequence-specific DNA analysis. Angew Chem Int Ed Engl, 121, 8826–8830.
Li, G.-Z., & Tian, F. (2013). Guanine-decorated graphene nanostructures for sensitive monitoring of neuron-specific enolase based on an enzyme-free electrocatalytic reaction. Analytical Sciences, 29, 1195–1201.
Pehlivan, Z. S., Torabfam, M., Kurt, H., Ow-Yang, C., Hildebrandt, N., & Yüce, M. (2019). Aptamer and nanomaterial based FRET biosensors: A review on recent advances (2014–2019). Microchimica Acta, 186, 563.
Hildebrandt, N., Spillmann, C. M., Algar, W. R., Pons, T., Stewart, M. H., Oh, E., Susumu, K., Diaz, S. A., Delehanty, J. B., Medintz, I. L. J. C. R. (2017). Energy transfer with semiconductor quantum dot bioconjugates: A versatile platform for biosensing, energy harvesting, and other developing applications. 117, 536-711.
Hunt, A., Dikin, D. A., Kurmaev, E. Z., Boyko, T. D., Bazylewski, P., Chang, G. S., & Moewes, A. (2012). Epoxide speciation and functional group distribution in graphene oxide paper-like materials. Chem Rev, 22, 3950–3957.
Yuan, H., Qi, J., Xing, C., An, H., Niu, R., Zhan, Y., Fan, Y., Yan, W., Li, R., Wang, B. J. A. F. M. (2015). Graphene‐oxide‐conjugated polymer hybrid materials for calmodulin sensing by using FRET strategy. 25, 4412-4418.
Hermann, T., & Patel, D. J. J. S. (2000). Adaptive recognition by nucleic acid aptamers. Science, 287, 820–825.
Liu, X., & Liu, J. J. V. (2021). Biosensors and sensors for dopamine detection., 2, 20200102.
Zhu, Y., Cai, Y., Xu, L., Zheng, L., Wang, L., Qi, B., Xu, C. (2015). Building an aptamer/graphene oxide FRET biosensor for one-step detection of bisphenol A. 7, 7492-7496.
Mao, B., Calatayud, D. G., Mirabello, V., Kuganathan, N., Ge, H., Jacobs, R. M., Shepherd, A. M., Martins, J. A. R., De La Serna, J. B., Hodges, B. (2017). Fluorescence‐lifetime imaging and super‐resolution microscopies shed light on the directed‐and self‐assembly of functional porphyrins onto carbon nanotubes and flat surfaces. 23, 9772
Duan, Y. F., Ning, Y., Song, Y., Deng, L. J. M. A. (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. 181, 647-653
Walsh, R., DeRosa, M. (2009). Retention of function in the DNA homolog of the RNA dopamine aptamer. 388, 732-735
Zhang, M., Yin, B.-C., Tan, W., Ye, B.-C. J. B., Bioelectronics (2011). A versatile graphene-based fluorescence “on/off” switch for multiplex detection of various targets. 26, 3260-3265.
Lee, J., Kim, J., Kim, S., Min, D.-H. J. A. (2016). Biosensors based on graphene oxide and its biomedical application. 105, 275-287.
Arvand, M., & Mirroshandel, A. A. J. B. (2017). Bioelectronics Highly-sensitive aptasensor based on fluorescence resonance energy transfer between l-cysteine capped ZnS quantum dots and graphene oxide sheets for the determination of edifenphos fungicide. Biosens Bioelectron, 96, 324–331.
Goud, K. Y., Sharma, A., Hayat, A., Catanante, G., Gobi, K. V., Gurban, A. M., Marty, J. (2016) Tetramethyl-6-carboxyrhodamine quenching-based aptasensing platform for aflatoxin B1: Analytical performance comparison of two aptamers. 508, 19-24.
Wang, Y., Kang, K., Wang, S., Kang, W., Cheng, C., Niu, L. M., & Guo, Z. (2020). A novel label-free fluorescence aptasensor for dopamine detection based on an Exonuclease III- and SYBR Green I- aided amplification strategy. Sensors and Actuators B: Chemical, 305, 127348.
Ren, L., Hang, X., Qin, Z., Zhang, P., Wang, W., Zhang, Y., & Jiang, L. (2020). Determination of dopamine by a label-free fluorescent aptasensor based on AuNPs and carbon quantum dots. Optik, 208, 163560.
Guo, T., Wu, C., Offenhäusser, A., Mayer, D. (2020). A novel ratiometric electrochemical biosensor based on a split aptamer for the detection of dopamine with logic gate operations. 217, 1900924
Jarczewska, M., Sheelam, S. R., Ziółkowski, R., & Górski, Ł. (2015). A label-free electrochemical DNA aptasensor for the detection of dopamine. Journal of The Electrochemical Society, 163, B26–B31.
Azadbakht, A., Roushani, M., Abbasi, A. R., Menati, S., & Derikvand, Z. (2016). A label-free aptasensor based on polyethyleneimine wrapped carbon nanotubes in situ formed gold nanoparticles as signal probe for highly sensitive detection of dopamine. Materials Science and Engineering: C, 68, 585–593.
Wang, Y., Li, Y., Tang, L., Lu, J., & Li, J. (2009). Application of graphene-modified electrode for selective detection of dopamine. Electrochemistry Communications, 11, 889–892.
Wei, B., Zhong, H., Wang, L., Liu, Y., Xu, Y., Zhang, J., Xu, C., He, L., & Wang, H. (2019). Facile preparation of a collagen-graphene oxide composite: A sensitive and robust electrochemical aptasensor for determining dopamine in biological samples. International Journal of Biological Macromolecules, 135, 400–406.
Liu, L., Xia, N., Meng, J.-J., Zhou, B.-B., & Li, S.-J. (2016). An electrochemical aptasensor for sensitive and selective detection of dopamine based on signal amplification of electrochemical-chemical redox cycling. Journal of Electroanalytical Chemistry, 775, 58–63.
Dalirirad, S., & Steckl, A. J. J. A. B. (2020). Lateral flow assay using aptamer-based sensing for on-site detection of dopamine in urine. Anal Biochem, 596, 113637.
Zheng, Y., Wang, Y., & Yang, X. (2011). Aptamer-based colorimetric biosensing of dopamine using unmodified gold nanoparticles. Sensors and Actuators B: Chemical, 156, 95–99.
Feng, J.-J., Guo, H., Li, Y.-F., Wang, Y.-H., Chen, W.-Y., & Wang, A.-J. (2013). Single molecular functionalized gold nanoparticles for hydrogen-bonding recognition and colorimetric detection of dopamine with high sensitivity and selectivity. ACS Applied Materials & Interfaces, 5, 1226–1231.
Xu, J., Li, Y., Wang, L., Huang, Y., Liu, D., Sun, R., Luo, J., & Sun, C. (2015). A facile aptamer-based sensing strategy for dopamine through the fluorescence resonance energy transfer between rhodamine B and gold nanoparticles. Dyes and Pigments, 123, 55–63.
Seto, D., Maki, T., Soh, N., Nakano, K., Ishimatsu, R., & Imato, T. (2012). A simple and selective fluorometric assay for dopamine using a calcein blue–Fe2+ complex fluorophore. Talanta, 94, 36–43.
Acknowledgements
This work was financially supported by Bioengineering Laboratory, Higher National School of Biotechnology, Constantine, Algeria. We thank the National Biotechnology Research Center for providing the microplate reader.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teniou, A., Rhouati, A. & Catanante, G. A Simple Fluorescent Aptasensing Platform Based on Graphene Oxide for Dopamine Determination. Appl Biochem Biotechnol 194, 1925–1937 (2022). https://doi.org/10.1007/s12010-022-03802-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03802-1