Abstract
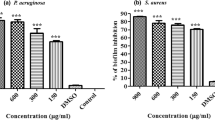
Leonurus sibiricus (Red verticilla, honeyweed) is a type of herbaceous plant predominantly found in Asian subcontinents as weed in crop fields and is widely used for treating diabetes, bronchitis, and menstrual irregularities. However, there is a dearth of study in the application of the plant phytocompounds for treating biofilm-associated chronic infections. The bioactive compounds mainly comprise of tri-terpenes, di-terpenes, phenolic acid, and flavonoids which may have potential role as antimicrobial and antibiofilm agents. Acute and chronic infection causing microbes usually form biofilm and develop virulence factors and antibiotic resistance through quorum sensing (QS). In this study, the bioactive compounds leosibirin, sibiricinone A, leosibirone A, leonotin, quercetin, lavandulifolioside, and myricetin were identified using GC–MS analysis. These were used for analyzing the antibiofilm and anti-quorum sensing activities (rhamnolipid, AHL assay, swarming motility assay) against the biofilm formed by Pseudomonas aeruginosa, the most significant nosocomial disease-causing bacteria. The compounds were able to bring about maximum inhibition in biofilm formation and QS. Although the antibiofilm activity of the phytoextract was found to be higher than that of individual phytocompounds at a concentration of 250 µg/mL, quercetin and myricetin showed highest antibiofilm activity against Pseudomonas aeruginosa, respectively, at MIC values of 135 µg/mL and 150 µg/mL against P aeruginosa. FT-IR study also revealed that the active ingredients were able to bring about the destruction of exopolysaccharides (EPS). These observations were further validated by molecular docking interactions that showed the active ingredients inhibit the functioning of QS sensing proteins by binding with them. It was observed that myricetin showed better interactions with the QS proteins of P. aeruginosa. Myricetin and quercetin show considerable inhibition of biofilm in comparison to the phytocompounds. Thus, the present study suggests that the active compounds from L. sibiricus can be used as an alternate strategy in inhibiting the biofilm formed by pathogenic organisms.








Similar content being viewed by others
Data Availability
Not applicable.
References
Cross, A., Allen, J. R., Burke, J., Ducel, G., Harris, A., John, J., & … Meers, P. (1983). Nosocomial infections due to Pseudomonas aeruginosa: Review of recent trends. Reviews of infectious diseases, 5(Suppl 5), S837–S845. https://doi.org/10.1093/clinids/5.supplement_5.s837
Donlan, R. M. (2002). Biofilms: Microbial life on surfaces. Emerging infectious diseases, 8(9), 881–890. https://doi.org/10.3201/eid0809.020063
Hall-Stoodley, L., Costerton, J. W., & Stoodley, P. (2004). Bacterial biofilms: From the natural environment to infectious diseases. Nature Reviews Microbiology, 2(2), 95–108. https://doi.org/10.1038/nrmicro821
Lahiri, D., Nag, M., Sheikh, H. I., Sarkar, T., Edinur, H., & atan, Siddhartha, P., & Ray, R. rani. (2021). Microbiologically synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Frontiers Microbiology, 12, 636588. https://doi.org/10.3389/fmicb.2021.636588
Nag, M., Lahiri, D., Sarkar, T., Ghosh, S., Dey, A., Edinur, H. A., & … Ray, R. R. (2021). Microbial fabrication of nanomaterial and its role in disintegration of exopolymeric matrices of biofilm. Frontiers in Chemistry, 9, 369. https://doi.org/10.3389/fchem.2021.690590
Breidenstein, E. B. M., de la Fuente-Núñez, C., & Hancock, R. E. W. (2011). Pseudomonas aeruginosa: All roads lead to resistance. Trends in microbiology, 19(8), 419–426. https://doi.org/10.1016/j.tim.2011.04.005
Liao, J., & Sauer, K. (2012). The MerR-like transcriptional regulator BrlR contributes to Pseudomonas aeruginosa biofilm tolerance. Journal of Bacteriology, 194(18), 4823–4836. https://doi.org/10.1128/JB.00765-12
Hurtuková, K., Fajstavrová, K., Rimpelová, S., Vokatá, B., Fajstavr, D., Kasálková, N. S., & … Slepička, P. (2021). Antibacterial properties of a honeycomb-like pattern with cellulose acetate and silver nanoparticles. Materials., 14(14), 4051. https://doi.org/10.3390/ma14144051
Škubník, J., Pavlíčková, V., Ruml, T., & Rimpelová, S. (2021). Current perspectives on taxanes: Focus on their bioactivity, delivery and combination therapy. Plants., 10(3), 569. https://doi.org/10.3390/plants10030569
Sayed, M. A., Alam, M. A., Islam, M. S., Ali, M. T., Ullah, M. E., Shibly, A. Z., … Hasan-Olive, M. M. (2016). Leonurus sibiricus L. (honeyweed): A review of its phytochemistry and pharmacology. Asian Pacific Journal of Tropical Biomedicine, 6(12), 1076–1080. https://doi.org/10.1016/j.apjtb.2016.10.003
Ahmed, F., Islam, M. A., & Rahman, M. M. (2006). Antibacterial activity of Leonurus sibiricus aerial parts. Fitoterapia, 77(4), 316–317. https://doi.org/10.1016/j.fitote.2006.03.005
Sitarek, P., Rijo, P., Garcia, C., Skała, E., Kalemba, D., Białas, A. J., … Śliwiński, T. (2017). Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxidative Medicine and Cellular Longevity, 2017, 7384061. https://doi.org/10.1155/2017/7384061
Quave, C. L., Estévez-Carmona, M., Compadre, C. M., Hobby, G., Hendrickson, H., Beenken, K. E., & Smeltzer, M. S. (2012). Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE, 7(1), e28737. https://doi.org/10.1371/journal.pone.0028737
Jeyaseelan, E. C., & Jashothan, P. T. J. (2012). In vitro control of Staphylococcus aureus (NCTC 6571) and Escherichia coli (ATCC 25922) by Ricinus communis L. Asian Pacific Journal of Tropical Biomedicine, 2(9), 717–721. https://doi.org/10.1016/S2221-1691(12)60216-0
Patra, J. K., Kim, E. S., Oh, K., Kim, H.-J., Kim, Y., & Baek, K.-H. (2014). Antibacterial effect of crude extract and metabolites of Phytolacca americana on pathogens responsible for periodontal inflammatory diseases and dental caries. BMC Complementary and Alternative Medicine, 14, 343. https://doi.org/10.1186/1472-6882-14-343
Lahiri, D., Nag, M., Sarkar, T., Dutta, B., & Ray, R. R. (2021). Antibiofilm activity of α-amylase from Bacillus subtilis and prediction of the optimized conditions for biofilm removal by response surface methodology (RSM) and artificial neural network (ANN). Applied Biochemistry and Biotechnology., 193(6), 1853–1872. https://doi.org/10.1007/s12010-021-03509-9
Teh, C. H., Nazni, W. A., Nurulhusna, A. H., Norazah, A., & Lee, H. L. (2017). Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiology, 17(1), 36. https://doi.org/10.1186/s12866-017-0936-3
Baishya, R., Bhattacharya, A., Mukherjee, M., Lahiri, D., & Banerjee, S. (2016). Establishment of a simple reproducible model for antibiotic sensitivity pattern study of biofilm forming staphylococcus aureus. Materials Today: Proceedings, 3(10, Part A), 3461–3466. https://doi.org/10.1016/j.matpr.2016.10.028
Lahiri, D., Nag, M., Dutta, B., Sarkar, T., & Ray, R. R. (2021). Artificial neural network and response surface methodology-mediated optimization of bacteriocin production by Rhizobium leguminosarum. Iranian Journal of Science and Technology, Transactions A: Science, 45,. https://doi.org/10.1007/s40995-021-01157-6
Yang, Y.-H., Lee, T.-H., Kim, J. H., Kim, E. J., Joo, H.-S., Lee, C.-S., & Kim, B.-G. (2006). High-throughput detection method of quorum-sensing molecules by colorimetry and its applications. Analytical Biochemistry, 356(2), 297–299. https://doi.org/10.1016/j.ab.2006.05.030
Caiazza, N. C., Shanks, R. M. Q., & O’Toole, G. A. (2005). Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. Journal of Bacteriology, 187(21), 7351–7361. https://doi.org/10.1128/JB.187.21.7351-7361.2005
Zhou, J., Bi, S., Chen, H., Chen, T., Yang, R., Li, M., & … Jia, A.-Q. (2017). Anti-biofilm and antivirulence activities of metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Frontiers in Microbiology, 8, 769. https://doi.org/10.3389/fmicb.2017.00769
Datta, S., Jana, D., Maity, T. R., Samanta, A., & Banerjee, R. (2016). Piper betle leaf extract affects the quorum sensing and hence virulence of Pseudomonas aeruginosa PAO1. 3 Biotech, 6(1), 18. https://doi.org/10.1007/s13205-015-0348-8
Ding, X., Peng, X.-J., Jin, B.-S., Xiao, M., Chen, J.-K., Li, B., & … Nie, M. (2015). Spatial distribution of bacterial communities driven by multiple environmental factors in a beach wetland of the largest freshwater lake in China. Frontiers in Microbiology, 6, 129. https://doi.org/10.3389/fmicb.2015.00129
Teanpaisan, R., Kawsud, P., Pahumunto, N., & Puripattanavong, J. (2017). Screening for antibacterial and antibiofilm activity in Thai medicinal plant extracts against oral microorganisms. Journal of Traditional and Complementary Medicine, 7(2), 172–177. https://doi.org/10.1016/j.jtcme.2016.06.007
Meade, H. M., Long, S. R., Ruvkun, G. B., Brown, S. E., & Ausubel, F. M. (1982). Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. Journal of Bacteriology, 149(1), 114–122. https://doi.org/10.1128/jb.149.1.114-122.1982
Consortium, S. G., Consortium, C. S. G., Consortium, N. S. G., Gräslund, S., Nordlund, P., Weigelt, J., … Gunsalus, K. C. (2008). Protein production and purification. Nature Methods, 5(2), 135–146. https://doi.org/10.1038/nmeth.f.202
Brunk, C. F., Jones, K. C., & James, T. W. (1979). Assay for nanogram quantities of DNA in cellular homogenates. Analytical Biochemistry, 92(2), 497–500. https://doi.org/10.1016/0003-2697(79)90690-0
Pati, S., Chatterji, A., Dash, B. P., Nelson, B. R., Sarkar, T., Shahimi, S., & … Acharya, D. (2020). Structural characterization and antioxidant potential of chitosan by γ-irradiation from the carapace of horseshoe crab. Polymers, 12(10), 2361. https://doi.org/10.3390/polym12102361
Sarkar, T., Bharadwaj, K. K., Salauddin, M., Pati, S., & Chakraborty, R. (2021). Phytochemical characterization, antioxidant, anti-inflammatory, anti-diabetic properties, molecular docking, pharmacokinetic profiling, and network pharmacology analysis of the major phytoconstituents of raw and differently dried Mangifera indica (Himsaga. Applied Biochemistry and Biotechnology. https://doi.org/10.1007/s12010-021-03669-8
Wang, Y., Xiao, J., Suzek, T. O., Zhang, J., Wang, J., & Bryant, S. H. (2009). PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Research, 37(Web Server issue), W623–33. https://doi.org/10.1093/nar/gkp456
Miean, K. H., & Mohamed, S. (2001). Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. Journal of Agricultural and Food Chemistry, 49(6), 3106–3112. https://doi.org/10.1021/jf000892m
Omar, S. H. (2018). Chapter 4 - Biophenols: Impacts and prospects in anti-Alzheimer drug discovery. In G. B. T.-D. and D. of N. A. from N. P. Brahmachari (Ed.), Natural Product Drug Discovery (pp. 103–148). Elsevier. https://doi.org/10.1016/B978-0-12-809593-5.00004-5
Tauchen, J., Huml, L., Rimpelova, S., & Jurášek, M. (2020). Flavonoids and related members of the aromatic polyketide group in human health and disease: Do they really work? Molecules, 25(17), 3846. https://doi.org/10.3390/molecules25173846
Ouyang, J., Sun, F., Feng, W., Sun, Y., Qiu, X., Xiong, L., & … Chen, Y. (2016). Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. Journal of Applied Microbiology, 120(4), 966–974. https://doi.org/10.1111/jam.13073
Mu, Y., Zeng, H., & Chen, W. (2021). Quercetin inhibits biofilm formation by decreasing the production of EPS and altering the composition of EPS in Staphylococcus epidermidis. Frontiers in Microbiology, 12, 251. https://doi.org/10.3389/fmicb.2021.631058
Soberón, J. R., Sgariglia, M. A., Sampietro, D. A., Quiroga, E. N., & Vattuone, M. A. (2007). Antibacterial activity of plant extracts from northwestern Argentina. Journal of Applied Microbiology, 102(6), 1450–1461. https://doi.org/10.1111/j.1365-2672.2006.03229.x
Silva, L. N., Da Hora, G. C. A., Soares, T. A., Bojer, M. S., Ingmer, H., Macedo, A. J., & Trentin, D. S. (2017). Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Scientific Reports, 7(1), 2823. https://doi.org/10.1038/s41598-017-02712-1
Spencer, J., Murphy, L. M., Conners, R., Sessions, R. B., & Gamblin, S. J. (2010). Crystal structure of the LasA virulence factor from Pseudomonas aeruginosa: Substrate specificity and mechanism of M23 metallopeptidases. Journal of Molecular Biology, 396(4), 908–923. https://doi.org/10.1016/j.jmb.2009.12.021
Leonard, P. G., Bezar, I. F., Sidote, D. J., & Stock, A. M. (2012). Identification of a hydrophobic cleft in the LytTR domain of AgrA as a locus for small molecule interactions that inhibit DNA binding. Biochemistry, 51(50), 10035–10043. https://doi.org/10.1021/bi3011785
Ghani, A. (1998). Medicinal plants of Bangladesh: Chemical constituents and uses.
Ahmad, S., Zahiruddin, S., Parveen, B., Basist, P., Parveen, A., & Gaurav, … Ahmad, M. (2021). Indian medicinal plants and formulations and their potential against COVID-19–preclinical and clinical research. Frontiers in Pharmacology, 11, 2470. https://doi.org/10.3389/fphar.2020.578970
Author information
Authors and Affiliations
Contributions
The authors contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
All work has been done under the guidelines of Institutional Ethics Committee MAKAUT: IEC-(18–19)/02 dated 28.12.2019.
Consent to Participate
All authors have their consent to participate.
Consent for Publication
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, S., Lahiri, D., Nag, M. et al. Analysis of Antibiofilm Activities of Bioactive Compounds from Honeyweed (Leonurus sibiricus) Against P. aeruginosa: an In Vitro and In Silico Approach. Appl Biochem Biotechnol 195, 5312–5328 (2023). https://doi.org/10.1007/s12010-021-03797-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03797-1