Abstract
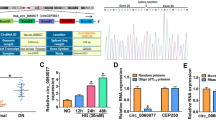
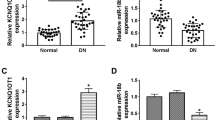
Long non-coding RNAs (lncRNAs) play crucial roles in the development of diabetic nephropathy (DN). Here, we explored the activity and mechanism of MIR503 host gene (MIR503HG) in high glucose (HG)-evoked cytotoxicity in HK-2 cells. MIR503HG, microRNA (miR)-497-5p, and C–C motif chemokine ligand 19 (CCL19) were quantified by quantitative real-time PCR (qRT-PCR) and western blot. The direct relationship between miR-497-5p and MIR503HG or CCL19 was confirmed by dual-luciferase reporter and RNA immunoprecipitation (RIP) assays. Cell viability and apoptosis were evaluated by XTT assay and flow cytometry, respectively. Our data showed that MIR503HG was overexpressed in HG-stimulated HK-2 cells. Knockdown of MIR503HG alleviated HG-evoked cell apoptosis, inflammation, and fibrosis in HK-2 cells. Mechanistically, MIR503HG regulated miR-497-5p expression via a binding site. MIR503HG depletion reduced HG-evoked cell apoptosis, inflammation, and fibrosis in HK-2 cells by up-regulating miR-497-5p. Moreover, miR-497-5p directly targeted and suppressed CCL19. MiR-497-5p-mediated suppression of CCL19 relieved HG-induced cell apoptosis, inflammation, and fibrosis in HK-2 cells. Furthermore, MIR503HG regulated CCL19 expression via miR-497-5p competition. Our findings identify a new MIR503HG/miR-497-5p/CCL19 network in the regulating HG-evoked cell apoptosis, inflammation, and fibrosis in HK-2 cells.







Similar content being viewed by others
Data Availability
Not applicable.
References
Umanath, K., & Lewis, J. B. (2018). Update on diabetic nephropathy: Core curriculum 2018. American Journal of Kidney Diseases, 71(6), 884–895.
Tziomalos, K., & Athyros, V. G. (2015). Diabetic nephropathy: New risk factors and improvements in diagnosis. The Review of Diabetic Studies, 12(1–2), 110–118.
Moreno, J. A., Gomez-Guerrero, C., Mas, S., Sanz, A. B., Lorenzo, O., Ruiz-Ortega, M., Opazo, L., Mezzano, S., & Egido, J. (2018). Targeting inflammation in diabetic nephropathy: A tale of hope. Expert Opinion on Investigational Drugs, 27(11), 917–930.
Calle P., Hotter G. (2020). Macrophage phenotype and fibrosis in diabetic nephropathy. Int J Mol Sci, 21(8).
Esteller, M. (2011). Non-coding RNAs in human disease. Nature Reviews Genetics, 12(12), 861–874.
Tay, Y., Rinn, J., & Pandolfi, P. P. (2014). The multilayered complexity of ceRNA crosstalk and competition. Nature, 505(7483), 344–352.
Salmena, L., Poliseno, L., Tay, Y., Kats, L., & Pandolfi, P. P. (2011). A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell, 146(3), 353–358.
Loganathan, T. S., Sulaiman, S. A., Abdul Murad, N. A., Shah, S. A., Abdul Gafor, A. H., Jamal, R., & Abdullah, N. (2020). Interactions among non-coding RNAs in diabetic nephropathy. Frontiers Pharmacology, 11, 191.
Yang, J., Shen, Y., Yang, X., Long, Y., Chen, S., Lin, X., Dong, R., & Yuan, J. (2019). Silencing of long noncoding RNA XIST protects against renal interstitial fibrosis in diabetic nephropathy via microRNA-93-5p-mediated inhibition of CDKN1A. American Journal of Physiology. Renal Physiology, 317(5), F1350-f1358.
Yu R., Zhang Y., Lu Z., Li J., Shi P., Li J. (2019). Long-chain non-coding RNA UCA1 inhibits renal tubular epithelial cell apoptosis by targeting microRNA-206 in diabetic nephropathy. Arch Physiol Biochem 1–9.
Muys B. R., Lorenzi J. C., Zanette D. L., Lima e Bueno Rde B., de Araújo L. F., Dinarte-Santos A. R., Alves C. P., Ramão A., de Molfetta G. A., Vidal D. O., Silva W. A., Jr. (2016). Placenta-enriched LincRNAs MIR503HG and LINC00629 decrease migration and invasion potential of JEG-3 cell line. PLoS One, 11(3), e0151560.
Dao, R., Wudu, M., Hui, L., Jiang, J., Xu, Y., Ren, H., & Qiu, X. (2020). Knockdown of lncRNA MIR503HG suppresses proliferation and promotes apoptosis of non-small cell lung cancer cells by regulating miR-489–3p and miR-625–5p. Pathology, Research and Practice, 216(3), 152823.
Xu, S., Zhai, S., Du, T., & Li, Z. (2020). LncRNA MIR503HG inhibits non-small cell lung cancer cell proliferation by inducing cell cycle arrest through the downregulation of cyclin D1. Cancer Management and Research, 12, 1641–1647.
Wei, J., Wang, Z., Zhong, C., Ding, H., Wang, X., & Lu, S. (2021). LncRNA MIR503HG promotes hypertrophic scar progression via miR-143-3p-mediated Smad3 expression. Wound Repair Regen, 29(5), 792–800.
Cheng D., Jiang S., Chen J., Li J., Ao L., Zhang Y. (2019). The increased lncRNA MIR503HG in preeclampsia modulated trophoblast cell proliferation, invasion, and migration via regulating matrix metalloproteinases and NF-κB signaling. Dis Markers, 20194976845.
Cao, X., & Fan, Q. L. (2020). LncRNA MIR503HG promotes high-glucose-induced proximal tubular cell apoptosis by targeting miR-503–5p/Bcl-2 pathway. Diabetes and Metabolic Syndrome Obesity Obesity, 13, 4507–4517.
Tang, J., Yao, D., Yan, H., Chen, X., Wang, L., & Zhan, H. (2019). The role of microRNAs in the pathogenesis of diabetic nephropathy. International Journal of Endocrinology, 2019, 8719060.
Lv, N., Li, C., Liu, X., Qi, C., & Wang, Z. (2019). miR-34b alleviates high glucose-induced inflammation and apoptosis in human HK-2 cells via IL-6R/JAK2/STAT3 signaling pathway. Medical Science Monitor, 25, 8142–8151.
Xue, M., Li, Y., Hu, F., Jia, Y. J., Zheng, Z. J., Wang, L., & Xue, Y. M. (2018). High glucose up-regulates microRNA-34a-5p to aggravate fibrosis by targeting SIRT1 in HK-2 cells. Biochemical and Biophysical Research Communications, 498(1), 38–44.
Liu, F., Zhang, S., Xu, R., Gao, S., & Yin, J. (2018). Melatonin attenuates endothelial-to-mesenchymal transition of glomerular endothelial cells via regulating miR-497/ROCK in diabetic nephropathy. Kidney & Blood Pressure Research, 43(5), 1425–1436.
Wang, J., & Zhao, S. M. (2021). LncRNA-antisense non-coding RNA in the INK4 locus promotes pyroptosis via miR-497/thioredoxin-interacting protein axis in diabetic nephropathy. Life Science, 264,
Zeng M., Liu J., Yang W., Zhang S., Liu F., Dong Z., Peng Y., Sun L., Xiao L. (2019). Multiple-microarray analysis for identification of hub genes involved in tubulointerstial injury in diabetic nephropathy. Journal of Cellular Physiology.
Yang, F., Cui, Z., Deng, H., Wang, Y., Chen, Y., Li, H., & Yuan, L. (2019). Identification of miRNAs-genes regulatory network in diabetic nephropathy based on bioinformatics analysis. Medicine (Baltimore), 98(27), e16225.
Sun, J., Wang, J., Lu, W., Xie, L., Lv, J., Li, H., & Yang, S. (2020). MiR-325-3p inhibits renal inflammation and fibrosis by targeting CCL19 in diabetic nephropathy. Clinical and Experimental Pharmacology and Physiology, 47(11), 1850–1860.
Iwakawa, H. O., & Tomari, Y. (2015). The functions of microRNAs: MRNA decay and translational repression. Trends in Cell Biology, 25(11), 651–665.
Yang, Y., Lv, X., Fan, Q., Wang, X., Xu, L., Lu, X., & Chen, T. (2019). Analysis of circulating lncRNA expression profiles in patients with diabetes mellitus and diabetic nephropathy: Differential expression profile of circulating lncRNA. Clinical Nephrology, 92(1), 25–35.
Lv, J., Wu, Y., Mai, Y., & Bu, S. (2020). Noncoding RNAs in Diabetic Nephropathy: Pathogenesis, Biomarkers, and Therapy. Journal of Diabetes Research, 2020, 3960857.
Chen, X., Shi, C., Wang, C., Liu, W., Chu, Y., Xiang, Z., Hu, K., Dong, P., & Han, X. (2017). The role of miR-497–5p in myofibroblast differentiation of LR-MSCs and pulmonary fibrogenesis. Scientific Reports, 7, 40958.
Chen, Q., Yan, J., Xie, W., Xie, W., Li, M., & Ye, Y. (2020). LncRNA LINC00641 sponges miR-497–5p to ameliorate neural injury induced by anesthesia via up-regulating BDNF. Frontiers in Molecular Neuroscience, 13, 95.
Chen, Y., Kuang, D., Zhao, X., Chen, D., Wang, X., Yang, Q., Wan, J., Zhu, Y., Wang, Y., Zhang, S., Wang, Y., Tang, Q., Masuzawa, M., Wang, G., & Duan, Y. (2016). miR-497–5p inhibits cell proliferation and invasion by targeting KCa3.1 in angiosarcoma. Oncotarget, 7(36), 58148–58161.
Gharib, E., Nasri, N. P., & Reza, Z. M. (2020). miR-497-5p mediates starvation-induced death in colon cancer cells by targeting acyl-CoA synthetase-5 and modulation of lipid metabolism. Journal of Cellular Physiology, 235(7–8), 5570–5589.
He, Z. (2019). LINC00473/miR-497-5p regulates esophageal squamous cell carcinoma progression through targeting PRKAA1. Cancer Biotherapy & Radiopharmaceuticals, 34(10), 650–659.
Hou, L., Shi, H., Wang, M., Liu, J., & Liu, G. (2019). MicroRNA-497-5p attenuates IL-1β-induced cartilage matrix degradation in chondrocytes via Wnt/β-catenin signal pathway. International Journal of Clinical and Experimental Pathology, 12(8), 3108–3118.
Ma J., Lin X., Chen C., Li S., Zhang S., Chen Z., Li D., Zhao F., Yang C., Yin C., Qiu W., Xiao Y., Zhang K., Miao Z., Yang T., Qian A. (2020). Circulating miR-181c-5p and miR-497–5p are potential biomarkers for prognosis and diagnosis of osteoporosis. The Journal of Clinical Endocrinology and Metabolism, 105(5).
Yan, Y., Chen, R., Wang, X., Hu, K., Huang, L., Lu, M., & Hu, Q. (2019). CCL19 and CCR7 expression, signaling pathways, and adjuvant functions in viral infection and prevention. Frontiers in Cell and Developmental Biology, 7, 212.
Adachi, K., Kano, Y., Nagai, T., Okuyama, N., Sakoda, Y., & Tamada, K. (2018). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nature Biotechnology, 36(4), 346–351.
Iida Y., Yoshikawa R., Murata A., Kotani H., Kazuki Y., Oshimura M., Matsuzaki Y., Harada M. (2020). Local injection of CCL19-expressing mesenchymal stem cells augments the therapeutic efficacy of anti-PD-L1 antibody by promoting infiltration of immune cells. The Journal for ImmunoTherapy of Cancer, 8(2).
Cheng, H. W., Onder, L., Cupovic, J., Boesch, M., Novkovic, M., Pikor, N., Tarantino, I., Rodriguez, R., Schneider, T., Jochum, W., Brutsche, M., & Ludewig, B. (2018). CCL19-producing fibroblastic stromal cells restrain lung carcinoma growth by promoting local antitumor T-cell responses. The Journal of Allergy and Clinical Immunology, 142(4), 1257-1271.e1254.
Rudnicki M., Perco P., B D. H., Leierer J., Heinzel A., Mühlberger I., Schweibert N., Sunzenauer J., Regele H., Kronbichler A., Mestdagh P., Vandesompele J., Mayer B., Mayer G. (2016). Renal microRNA- and RNA-profiles in progressive chronic kidney disease. European Journal of Clinical Investigation, 46(3), 213-226.
Sato, Y., & Yanagita, M. (2017). Resident fibroblasts in the kidney: A major driver of fibrosis and inflammation. Inflammation and Regeneration, 37, 17.
Iijima, N., Yanagawa, Y., Clingan, J. M., & Onoé, K. (2005). CCR7-mediated c-Jun N-terminal kinase activation regulates cell migration in mature dendritic cells. International Immunology, 17(9), 1201–1212.
Yang, H., Kan, Q. E., Su, Y., & Man, H. (2019). Long non-coding RNA CASC2 improves diabetic nephropathy by inhibiting JNK pathway. Experimental and Clinical Endocrinology & Diabetes, 127(8), 533–537.
Qiao, Y., Gao, K., Wang, Y., Wang, X., & Cui, B. (2017). Resveratrol ameliorates diabetic nephropathy in rats through negative regulation of the p38 MAPK/TGF-β1 pathway. Experimental and Therapeutic Medicine, 13(6), 3223–3230.
Qiu, F., Zhang, M. R., Zhou, Z., Pu, J. X., & Zhao, X. J. (2019). lncRNA MIR503HG functioned as a tumor suppressor and inhibited cell proliferation, metastasis and epithelial-mesenchymal transition in bladder cancer. Journal of Cellular Biochemistry, 120(6), 10821–10829.
Wang, H., Liang, L., Dong, Q., Huan, L., He, J., Li, B., Yang, C., Jin, H., Wei, L., Yu, C., Zhao, F., Li, J., Yao, M., Qin, W., Qin, L., & He, X. (2018). Long noncoding RNA miR503HG, a prognostic indicator, inhibits tumor metastasis by regulating the HNRNPA2B1/NF-κB pathway in hepatocellular carcinoma. Theranostics, 8(10), 2814–2829.
Monteiro, J. P., Rodor, J., Caudrillier, A., Scanlon, J. P., Spiroski, A. M., Dudnakova, T., Pflüger-Müller, B., Shmakova, A., von Kriegsheim, A., Deng, L., Taylor, R. S., Wilson-Kanamori, J. R., Chen, S. H., Stewart, K., Thomson, A., Mitić, T., McClure, J. D., Iynikkel, J., Hadoke, P. W. F., … Baker, A. H. (2021). MIR503HG loss promotes endothelial-to-mesenchymal transition in vascular disease. Circulation Research, 128(8), 1173–1190.
Author information
Authors and Affiliations
Contributions
Danping Zhang designed and performed the research; Danping Zhang, Xiaoxiao Chen, and Dan Zheng analyzed the data; Danping Zhang wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Written informed consents were obtained from all participants, and this study was permitted by the Ethics Committee of Wenling First People’s Hospital of Zhejiang Province.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Chen, X. & Zheng, D. A Novel MIR503HG/miR-497-5p/CCL19 Axis Regulates High Glucose-Induced Cell Apoptosis, Inflammation, and Fibrosis in Human HK-2 Cells. Appl Biochem Biotechnol 194, 2061–2076 (2022). https://doi.org/10.1007/s12010-021-03776-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03776-6