Abstract
MiR-143/145 cluster is a novel transcriptional target of many signaling pathways, with variations within this cluster contributed to the risk of multiple diseases. To date, no data regarding the link between miR143/145 cluster polymorphisms and the risk of developing chronic kidney disease (CKD) has been reported. Hence, we aimed to examine such association in a population of Iranian ancestry. In this preliminary study, 276 CKD patients and 300 unrelated age and sex-matched healthy controls were recruited. Genotyping was performed by PCR-RFLP and allele-specific-PCR methods. Computational analyses were performed to predict the potential effects of the variants. Our findings indicated that rs41291957, rs12659504, and rs353292 polymorphisms were positively associated with CKD, while rs4705342 and rs4705343 polymorphisms demonstrated a significant negative association with the disease. Moreover, a significant association was observed between CC + TC and TT genotypes and CKD stages. We found that AACTT, AATTC, AATTT, GATTC, GATTT, and GGCTT haplotypes significantly enhanced the risk of CKD compared with the Grs41291957AArs12659504Crs353292Trs4705342Trs4705343 haplotype. Computational analysis showed that rs353292, rs4705342, and rs4705343 might alter the binding of the transcription factors in this gene cluster. We found that miR-143/145 cluster polymorphisms were associated with CKD risk in a sample of the Iranian population. Replicated studies on different ethnicities are necessary to investigate the association between these promoter variants and clinical outcomes.
Graphical abstract


Similar content being viewed by others
Data Availability
Data would be available within the article or its supplementary materials.
References
Datta, S. K., Kumar, V., Pathak, R., Tripathi, A. K., Ahmed, R. S., Kalra, O. P., & Banerjee, B. D. (2010). Association of glutathione S-transferase M1 and T1 gene polymorphism with oxidative stress in diabetic and nondiabetic chronic kidney disease. Renal Failure, 32(10), 1189–1195.
Yoshida, T., Kato, K., Fujimaki, T., Yokoi, K., Oguri, M., Watanabe, S., Metoki, N., Yoshida, H., Satoh, K., Aoyagi, Y., Nishigaki, Y., Tanaka, M., Nozawa, Y., & Yamada, Y. (2009). Association of a polymorphism of the apolipoprotein E gene with chronic kidney disease in Japanese individuals with metabolic syndrome. Genomics., 93(3), 221–226.
Wong, C., Kanetsky, P., & Raj, D. (2008). Genetic polymorphisms of the RAS-cytokine pathway and chronic kidney disease. Pediatric Nephrology, 23(7), 1037–1051.
Sargazi, F. M., Alidadi, A., Taheri, H., Nia, M. H., Sargazi, S., Saravani, R., et al. (2020). Functional miR29a gene polymorphism enhanced the risk of chronic kidney disease in an Iranian population: a preliminary case-control study and bioinformatics analyses. Meta Gene., 100755.
Eddy, A. A. (2005). Progression in chronic kidney disease. Advances in Chronic Kidney Disease, 12(4), 353–365.
Proctor, R., Kumar, N., Stein, A., Moles, D., & Porter, S. (2005). Oral and dental aspects of chronic renal failure. Journal of Dental Research, 84(3), 199–208.
Rajapurkar, M. M., John, G. T., Kirpalani, A. L., Abraham, G., Agarwal, S. K., Almeida, A. F., Gang, S., Gupta, A., Modi, G., Pahari, D., Pisharody, R., Prakash, J., Raman, A., Rana, D. S., Sharma, R. K., Sahoo, R. N., Sakhuja, V., Tatapudi, R. R., & Jha, V. (2012). What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrology, 13(1), 10.
Mahajan, S., Kalra, O. P., Tripathi, A. K., Ahuja, G., & Kalra, V. (2005). Phagocytic polymorphonuclear function in patients with progressive uremia and the effect of acute hemodialysis. Renal Failure, 27(4), 357–360.
Peralta, C. A., Katz, R., DeBoer, I., Ix, J., Sarnak, M., Kramer, H., Siscovick, D., Shea, S., Szklo, M., & Shlipak, M. (2011). Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. Journal of the American Society of Nephrology, 22(7), 1327–1334.
Hogg, R. J., Furth, S., Lemley, K. V., Portman, R., Schwartz, G. J., Coresh, J., Balk, E., Lau, J., Levin, A., Kausz, A. T., Eknoyan, G., & Levey, A. S. (2003). National kidney foundation's kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics., 111(6), 1416–1421.
Xin, M., Small, E. M., Sutherland, L. B., Qi, X., McAnally, J., Plato, C. F., Richardson, J. A., Bassel-Duby, R., & Olson, E. N. (2009). MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes & Development, 23(18), 2166–2178.
Cheng, G. (2015). Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Advanced Drug Delivery Reviews, 81, 75–93.
Wang, H., Peng, W., Shen, X., Huang, Y., Ouyang, X., & Dai, Y. (2012). Circulating levels of inflammation-associated miR-155 and endothelial-enriched miR-126 in patients with end-stage renal disease. Brazilian Journal of Medical and Biological Research, 45(12), 1308–1314.
Trionfini, P., Benigni, A., & Remuzzi, G. (2015). MicroRNAs in kidney physiology and disease. Nature Reviews. Nephrology, 11(1), 23–33.
Chandrasekaran, K., Karolina, D. S., Sepramaniam, S., Armugam, A., Wintour, E. M., Bertram, J. F., et al. (2012). Role of microRNAs in kidney homeostasis and disease. Kidney International, 81(7), 617–627.
Trakooljul, N., Hicks, J., & Liu, H. C. (2010). Identification of target genes and pathways associated with chicken microRNA miR-143. Animal Genetics, 41(4), 357–364.
Li, L., Pan, X., Li, Z., Bai, P., Jin, H., Wang, T., Song, C., Zhang, L., & Gao, L. (2013). Association between polymorphisms in the promoter region of miR-143/145 and risk of colorectal cancer. Human Immunology, 74(8), 993–997.
Chu, H., Zhong, D., Tang, J., Li, J., Xue, Y., Tong, N., Qin, C., Yin, C., Zhang, Z., & Wang, M. (2016). A functional variant in miR-143 promoter contributes to prostate cancer risk. Archives of Toxicology, 90(2), 403–414.
Liang, Y., Sun, R., Li, L., Yuan, F., Liang, W., Wang, L., et al. (2015). A functional polymorphism in the promoter of MiR-143/145 is associated with the risk of cervical squamous cell carcinoma in Chinese women: a case–control study. Medicine, 94(31), e1289.
Zhang, K., Mir, S. A., Hightower, C. M., Miramontes-Gonzalez, J. P., Maihofer, A. X., Chen, Y., Mahata, S. K., Nievergelt, C. M., Schork, N. J., Freedman, B. I., Vaingankar, S. M., & O'Connor, D. T. (2015). Molecular mechanism for hypertensive renal disease: differential regulation of chromogranin A expression at 3′-untranslated region polymorphism C+ 87 T by MicroRNA-107. Journal of the American Society of Nephrology, 26(8), 1816–1825.
Davey Smith, G., & Ebrahim, S. (2003). 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology, 32(1), 1–22.
Eknoyan, G., Lameire, N., Eckardt, K., Kasiske, B., Wheeler, D., Levin, A., et al. (2013). KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International, 3(1), 5–14.
Sargazi, S., Nia, M. H., Saravani, R., Shahroudi, M. J., Jahantigh, D., & Shakiba, M. (2020). IGF2BP2 polymorphisms as genetic biomarkers for either schizophrenia or type 2 diabetes mellitus: a case-control study. Gene Reports., 20, 100680.
Messeguer, X., Escudero, R., Farré, D., & Nuñez, O. (2002). Martı́nez J, Albà MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics., 18(2), 333–334.
Sloutskin, A., Danino, Y. M., Orenstein, Y., Zehavi, Y., Doniger, T., Shamir, R., & Juven-Gershon, T. (2015). ElemeNT: a computational tool for detecting core promoter elements. Transcription., 6(3), 41–50.
Crooks, G. E., Hon, G., Chandonia, J.-M., & Brenner, S. E. (2004). WebLogo: a sequence logo generator. Genome Research, 14(6), 1188–1190.
Pan, X., Xiao, X., Qin, H., Zhang, Z., Li, Z., Gao, L., et al. (2016). MicroRNA variants and colorectal cancer risk: a meta-analysis. Genetics and Molecular Research, 15(3).
Misra, M. K., Pandey, S. K., Kapoor, R., Sharma, R. K., & Agrawal, S. (2014). Genetic variants of MicroRNA-related genes in susceptibility and prognosis of end-stage renal disease and renal allograft outcome among north Indians. Pharmacogenetics and Genomics, 24(9), 442–450.
Chiang, C.-K., Hsu, S.-P., Wu, C.-T., Huang, J.-W., Cheng, H.-T., Chang, Y.-W., Hung, K. Y., Wu, K. D., & Liu, S. H. (2011). Endoplasmic reticulum stress implicated in the development of renal fibrosis. Molecular Medicine, 17(11), 1295–1305.
Shu, S., Zhu, J., Liu, Z., Tang, C., Cai, J., & Dong, Z. (2018). Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine., 37, 269–280.
Nassirpour, R., Raj, D., Townsend, R., & Argyropoulos, C. (2016). MicroRNA biomarkers in clinical renal disease: from diabetic nephropathy renal transplantation and beyond. Food and Chemical Toxicology, 98(Pt A), 73–88.
Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice J-M, et al. (2013). Increased circulating miR-21 levels are associated with kidney fibrosis PloS one. 8(2):e58014.
Chau, B. N., Xin, C., Hartner, J., Ren, S., Castano, A. P., Linn, G., et al. (2012). MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Science Translational Medicine, 4(121), 121ra18–121ra18.
Morizane, R., Fujii, S., Monkawa, T., Hiratsuka, K., Yamaguchi, S., Homma, K., et al. (2014). miR-34c attenuates epithelial-mesenchymal transition and kidney fibrosis with ureteral obstruction. Scientific Reports, 4, 4578.
Tang, O., Chen, X.-M., Shen, S., Hahn, M., & Pollock, C. A. (2013). MiRNA-200b represses transforming growth factor-β1-induced EMT and fibronectin expression in kidney proximal tubular cells. American Journal of Physiology. Renal Physiology, 304(10), F1266–F1F73.
He, J., Xu, Y., Koya, D., & Kanasaki, K. (2013). Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clinical and Experimental Nephrology, 17(4), 488–497.
Poli, V., Seclì, L., & Avalle, L. (2020). The Microrna-143/145 Cluster in Tumors: A Matter of Where and When. Cancers., 12(3), 708.
Bär, C., Thum, T., de Gonzalo-Calvo, D. (2019). Circulating miRNAs as mediators in cell-to-cell communication. Future Medicine 11(2):epi-20180183..
Rangrez, A. Y., Massy, Z. A., Metzinger-Le Meuth, V., & Metzinger, L. (2011). miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circulation. Cardiovascular Genetics, 4(2), 197–205.
Bradshaw, G., Haupt, L. M., Aquino, E. M., Lea, R. A., Sutherland, H. G., & Griffiths, L. R. (2019). Single nucleotide polymorphisms in MIR143 contribute to protection against Non-Hodgkin Lymphoma (NHL) in Caucasian populations. Genes., 10(3), 185.
Yang, X., Li, X., Quan, X., Li, H., Hao, X., Jiang, M., & Zhou, B. (2019). Association between two polymorphisms in the promoter region of miR-143/miR-145 and the susceptibility of lung cancer in northeast Chinese nonsmoking females. DNA and Cell Biology, 38(8), 814–823.
Su, J., Liang, H., Yao, W., Wang, N., Zhang, S., Yan, X., et al. (2014). MiR-143 and MiR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer. PLoS One, 9(12), e114420.
Qin, J., Wang, F., Jiang, H., Xu, J., Jiang, Y., & Wang, Z. (2015). MicroRNA-145 suppresses cell migration and invasion by targeting paxillin in human colorectal cancer cells. International Journal of Clinical and Experimental Pathology, 8(2), 1328–1340.
Bahmanpour, Z., Sheervalilou, R., Choupani, J., Shekari Khaniani, M., Montazeri, V., & Mansoori, D. S. (2019). A new insight on serum microRNA expression as novel biomarkers in breast cancer patients. Journal of Cellular Physiology, 234(11), 19199–19211.
Harvey, S. J., Jarad, G., Cunningham, J., Goldberg, S., Schermer, B., Harfe, B. D., McManus, M. T., Benzing, T., & Miner, J. H. (2008). Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. Journal of the American Society of Nephrology, 19(11), 2150–2158.
Chen, N. X., Kiattisunthorn, K., O'Neill, K. D., Chen, X., Moorthi, R. N., & Gattone, V. H. (2013). Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD). PLoS One, 8(5), e64558.
Taïbi, F., Metzinger-Le Meuth, V., M'Baya-Moutoula, E., Seif el Islam Djelouat, M., Louvet, L., Bugnicourt, J.-M., et al. (2014). Possible involvement of microRNAs in vascular damage in experimental chronic kidney disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1842(1), 88–98.
Brigant, B., Metzinger-Le Meuth, V., Massy, Z. A., McKay, N., Liabeuf, S., Pelletier, M., et al. (2017). Serum microRNAs are altered in various stages of chronic kidney disease: a preliminary study. Clinical Kidney Journal, 10(1), 30–37.
Fan, P.-C., Chen, C.-C., Peng, C.-C., Chang, C.-H., Yang, C.-H., Yang, C., Chu, L. J., Chen, Y. C., Yang, C. W., Chang, Y. S., & Chu, P. H. (2019). A circulating miRNA signature for early diagnosis of acute kidney injury following acute myocardial infarction. Journal of Translational Medicine, 17(1), 139.
Cheng, T., Hu, C., Yang, H., Cao, L., & An, J. (2014). Transforming growth factor-β-induced miR-143 expression in regulation of non-small cell lung cancer cell viability and invasion capacity in vitro and in vivo. International Journal of Oncology, 45(5), 1977–1988.
Climent, M., Quintavalle, M., Miragoli, M., Chen, J., Condorelli, G., & Elia, L. (2015). TGFβ triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circulation Research, 116(11), 1753–1764.
Meng, X.-M., Tang, P. M.-K., Li, J., & Lan, H. Y. (2015). TGF-β/Smad signaling in renal fibrosis. Frontiers in Physiology, 6, 82.
Zhong, X., Chung, A. C., Chen, H.-Y., Meng, X.-M., & Lan, H. Y. (2011). Smad3-mediated upregulation of miR-21 promotes renal fibrosis. Journal of the American Society of Nephrology, 22(9), 1668–1681.
Acknowledgments
We wish to thank Dr. Hamed Taheri for the samples provided.
Funding
This study was financially supported by a grant from Zahedan University of Medical Sciences, Zahedan, Iran (Grant No. 9457).
Author information
Authors and Affiliations
Contributions
SS and RS conceived and designed the experiments. MHN and RS analyzed the data. SB and FMS performed the genotyping. SS and SM wrote the first draft of the manuscript. SS, AA, and RS contributed to the writing of the manuscript. SS and RS made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
Ethical approval was obtained from the local Ethics committee of Zahedan University of Medical Sciences (Ethical code: IR.ZAUMS.REC.1398.172), in accordance with the Declaration of Helsinki.
Consent to Participate
Written informed consent was taken from all the participants.
Consent to Publish
The authors grant the publisher exclusive publication and dissemination rights.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
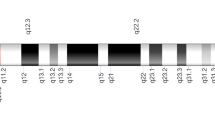
Supplementary Fig. 1
Schematic diagram of chromosome 5 and the locations of studied variants (TIF 47 kb)
Supplementary Fig. 2
LD analysis of rs41291957, rs12659504, rs353292, rs4705342, and rs4705343. Poor linkage disequilibrium was observed between rs41291957 and rs12659504 (TIF 92 kb)
Supplementary Fig. 3
The impact of rs353292 C/T (A) and rs4705342 C/T (B) on transcription factor binding sites through promoter of miR-143. A new C/EBPbeta binding site is created due to the presence of the T allele in the rs353292 position. T allele of rs4705342 creates a new XBP-1 binding site in the miR-143 promoter region. Other studied variants did not alter transcription factor binding sites (TIF 69 kb)
Supplementary Fig. 4
The results of core promoter motifs analysis performed by the elemeNT server for T allele of rs4705343 (A) and C allele of rs4705343 (B) allele. An alternate TATA box was expanded by the T allele (TIF 60 kb)

ESM 5
(DOCX 12 kb)
ESM 6
(DOCX 13 kb)
ESM 7
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Sargazi, S., Heidari Nia, M., Mirani Sargazi, F. et al. Functional miR143/145 Cluster Variants and Haplotypes Are Associated with Chronic Kidney Disease: a Preliminary Case-Control Study and Computational Analyses. Appl Biochem Biotechnol 193, 1532–1544 (2021). https://doi.org/10.1007/s12010-021-03489-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03489-w