Abstract
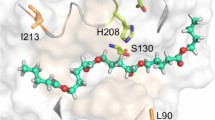
Cutinase-like enzymes (CLEs) are bi-functional hydrolases, which share the conserved catalytic site of lipase and consensus pentapeptide sequence of cutinase. Here, we have genetically replaced the canonical amino acids (CAA) by their non-canonical fluorinated surrogates to biosynthesize a novel class of congener biocatalyst for esterification of polymeric carbohydrate with long-chain fatty acid. It is a new enzyme-engineering approach used to manipulate industrially relevant biocatalyst through genetic incorporation of new functionally encoded non-canonical amino acids (NCAA). Global fluorination of CLE improved its catalytic, functional, and structural stability. Molecular docking studies confirmed that the fluorinated CLE (FCLE) had developed a binding affinity towards different fatty acids compared with the parent CLE. Importantly, FCLE could catalyze starch oleate synthesis in 24 h with a degree of substitution of 0.3 ± 0.001. Biophysical and microscopic analysis substantiated the efficient synthesis of the ester by FCLE. Our data represent the first step in the generation of an industrially relevant fluorous multifunctional enzyme for facile synthesis of high fatty acid starch esters.





Similar content being viewed by others
References
Lu, X., Luo, Z., Yu, S., & Fu, X. (2012). Lipase-catalyzed synthesis of starch palmitate in mixed ionic liquids. Journal of Agricultural and Food Chemistry, 60(36), 9273–9279.
Mayilvahanan, A., Ramchary, A., Niraikulam, A., Marichetti Kuppuswami, G., & NumbiRamudu, K. (2019). A green process for starch oleate synthesis by Cryptococcus sp. MTCC 5455 lipase and its potential as an emulsifying agent. Starch, 71(1-2), 1700325.
Tian, S., Chen, Y., Chen, Z., Yang, Y., & Wang, Y. (2018). Preparation and characteristics of starch esters and its effects on dough physicochemical properties. Journal of Food Quality, 7, 1395978.
Wang, Y., Xin, J., Shi, J., & Xia, C. (2013). Lipase-catalyzed esterification of corn-starch with oleic acid in solvent-free system. Biotechnology: An Indian Journal, 8(12), 1717–1725.
Ferrario, V., Pellis, A., Cespugli, M., Guebitz, G. M., & Gardossi, L. (2016). Nature inspired solutions for polymers: will cutinase enzymes make polyesters and polyamides greener? Catalysts, 6(12), 205.
Acevedo-Rocha, C. G., Hoesl, M. G., Nehring, S., Royter, M., Wolschner, C., Wiltschi, B., Antranikian, G., & Budisa, N. (2013). Non-canonical amino acids as a useful synthetic biological tool for lipase-catalysed reactions in hostile environments. Catalysis Science & Technology, 3(5), 1198–1201.
Zheng, C., Li, Z., Su, J., Zhang, R., Liu, C., & Zhao, M. (2012). Characterization and emulsifying property of a novel bioemulsifier by Aeribacillus pallidus YM-1. Journal of Applied Microbiology, 113(1), 44–51.
Voller, J. S., & Budisa, N. (2017). Coupling genetic code expansion and metabolic engineering for synthetic cells. Current Opinion in Biotechnology, 48, 1–7.
Pandurangan, S., Meganathan, I., Ragavan, S., Ramudu, K. N., Shanmugam, E., Shanmugam, G., & Niraikulam, A. (2019). Engineering of a skin-fiber-opening enzyme for sulfide-free leather beam house operation through xenobiology. Green Chemistry, 21(8), 2070–2081.
Salwiczek, M., Nyakatura, E. K., Gerling, U. I., Ye, S., & Koksch, B. (2012). Fluorinated amino acids: compatibility with native protein structures and effects on protein–protein interactions. Chemical Society Reviews, 41(6), 2135–2171.
Biava, H., & Budisa, N. (2014). Evolution of fluorinated enzymes: an emerging trend for biocatalyst stabilization. Engineering in Life Sciences, 14(4), 340–351.
Budisa, N., Wenger, W., & Wiltschi, B. (2010). Residue-specific global fluorination of Candida antarctica lipase B in Pichia pastoris. Molecular BioSystems, 6(9), 1630–1639.
Deepankumar, K., Shon, M., Nadarajan, S. P., Shin, G., Mathew, S., Ayyadurai, N., Kim, B. G., Choi, S. H., Lee, S. H., & Yun, H. (2014). Enhancing thermostability and organic solvent tolerance of ω-transaminase through global incorporation of fluorotyrosine. Advanced Synthesis & Catalysis, 356(5), 993–998.
Aarthy, M., Saravanan, P., Ayyadurai, N., Gowthaman, M. K., & Kamini, N. R. (2016). A two step process for production of omega 3-polyunsaturated fatty acid concentrates from sardine oil using Cryptococcus sp. MTCC 5455 lipase. Journal of Molecular Catalysis B: Enzymatic, 125, 25–33.
Drienovska, I., & Roelfes, G. (2020). Expanding the enzyme universe with genetically encoded unnatural amino acids. Nature Catalysis, 3(3), 193–202.
Lin, X., Yu, A. C. S., & Chan, T. F. (2017). Efforts and challenges in engineering the genetic code. Life, 7(1), 12.
Ravikumar, Y., Nadarajan, S. P., Yoo, T. H., Lee, C. S., & Yun, H. (2015). Unnatural amino acid mutagenesis-based enzyme engineering. Trends in Biotechnology, 33(8), 462–470.
Sambrook, J., & Russell, D. (2001). Molecular cloning, a laboratory manual. Volume 3. Third Editionth edition.
Zhang, Y., Gan, T., Hu, H., Huang, Z., Huang, A., Zhu, Y., Feng, Z., & Yang, M. (2014). A green technology for the preparation of high fatty acid starch esters: solid-phase synthesis of starch laurate assisted by mechanical activation with stirring ball mill as reactor. Industrial & Engineering Chemistry Research, 53(6), 2114–2120.
Aarthy, M., Puhazhselvan, P., Aparna, R., George, A. S., Gowthaman, M. K., Ayyadurai, N., Masaki, K., Nakajima-Kambe, T., & Kamini, N. R. (2018). Growth associated degradation of aliphatic-aromatic copolyesters by Cryptococcus sp. MTCC 5455. Polymer Degradation and Stability, 152, 20–28.
Kodama, Y., Masaki, K., Kondo, H., Suzuki, M., Tsuda, S., Nagura, T., Shimba, N., Suzuki, E. I., & Iefuji, H. (2009). Crystal structure and enhanced activity of a cutinase-like enzyme from Cryptococcus sp. strain S-2. Proteins: Structure, Function, and Bioinformatics, 77(3), 710–717.
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R., & Sherman, W. (2013). Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. Journal of Computer-aided Molecular Design, 27(3), 221–234.
Halgren, T. A., Murphy, R. B., Friesner, R. A., Beard, H. S., Frye, L. L., Pollard, W. T., & Banks, J. L. (2004). Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. Journal of Medicinal Chemistry, 47(7), 1750–1759.
Shelley, J. C., Cholleti, A., Frye, L. L., Greenwood, J. R., Timlin, M. R., & Uchimaya, M. (2007). Epik: a software program for pK a prediction and protonation state generation for drug-like molecules. Journal of Computer-Aided Molecular Design, 21(12), 681–691.
Isobe, K., Akiba, T., & Yamaguchi, S. (1988). Crystallization and characterization of lipase from Penicillium cyclopium. Agricultural and Biological Chemistry, 52(1), 41–47.
Raghavan, S. S., Niraikulam, A., & Gunasekaran, K. (2019). Side chain torsion dictates planarity and ionizability of green fluorescent protein’s chromophore leading to spectral perturbations. Journal of Biomolecular Structure and Dynamics, 37(17), 4450–4459.
Singh, A., Upadhyay, V., Upadhyay, A. K., Singh, S. M., & Panda, A. K. (2015). Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microbial Cell Factories, 14(1), 1–10.
Cirino, P. C., Tang, Y., Takahashi, K., Tirrell, D. A., & Arnold, F. H. (2003). Global incorporation of norleucine in place of methionine in cytochrome P450 BM-3 heme domain increases peroxygenase activity. Biotechnology and Bioengineering, 83(6), 729–734.
Ring, M., Armitage, I. M., & Huber, R. E. (1985). m-Fluorotyrosine substitution in β-galactosidase; evidence for the existence of a catalytically active tyrosine. Biochemical and Biophysical Research Communications, 131(2), 675–680.
Ahmad, S., & Rao, N. M. (2009). Thermally denatured state determines refolding in lipase: mutational analysis. Protein Science, 18(6), 1183–1196.
Han, F., Gao, C., Liu, M., Huang, F., & Zhang, B. (2013). Synthesis, optimization and characterization of acetylated corn starch with the high degree of substitution. International Journal of Biological Macromolecules, 59, 372–376.
Kshirsagar, A. C., & Singhal, R. S. (2007). Optimization of starch oleate derivatives from native corn and hydrolyzed corn starch by response surface methodology. Carbohydrate Polymers, 69(3), 455–461.
Zarski, A., Ptak, S., Siemion, P., & Kapusniak, J. (2016). Esterification of potato starch by a biocatalysed reaction in an ionic liquid. Carbohydrate Polymers, 137, 657–663.
Wang, Y., Xin, J., Shi, J., Wu, W., & Xia, C. (2014). A kinetic study of starch palmitate synthesis by immobilized lipase-catalyzed esterification in solvent free system. Journal of Molecular Catalysis B: Enzymatic, 101, 73–79.
Horchani, H., Chaâbouni, M., Gargouri, Y., & Sayari, A. (2010). Solvent-free lipase-catalyzed synthesis of long-chain starch esters using microwave heating: optimization by response surface methodology. Carbohydrate Polymers, 79(2), 466–474.
Tupa, M. V., Arroyo, S., Herrera, M. L., & Foresti, M. L. (2018). Production of esterified starches with increased resistant starch content by an α-hydroxy acid-catalyzed route. Starch, 70(5-6), 1700155.
Acknowledgments
We thank the Director, CSIR-CLRI, for his support during the project. We also thank ICMR (File No: 5/3/8/4/2019-ITR) for research support.
Funding
We acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of Senior Research Fellowship to Ms. Sisila V and the Indian Council of Medical Research (ICMR) for Research Associate to Dr. M. Aarthy. The authors are grateful to CSIR-CLRI for funding this research through the “Major Laboratory Project - Biotechnology division.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1899 kb)
Rights and permissions
About this article
Cite this article
Sisila, V., Puhazhselvan, P., Aarthy, M. et al. Esterification of Polymeric Carbohydrate Through Congener Cutinase-Like Biocatalyst. Appl Biochem Biotechnol 193, 19–32 (2021). https://doi.org/10.1007/s12010-020-03415-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03415-6