Abstract
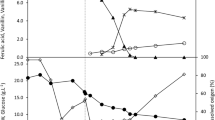
Amycolatopsis sp. ATCC 39116 catabolizes ferulic acid by the non-oxidative deacetylation and β-oxidation pathways to produce vanillin and vanillic acid, respectively. In submerged culture, vanillin productivity decreased more than 8-fold, when ferulic, p-coumaric, and caffeic acids were employed in pre-cultures of the microorganism in order to activate the ferulic acid catabolic pathways, resulting in a carbon redistribution since vanillic acid and guaiacol productivities increased more than 5-fold compared with control. In contrast, in surface culture, the effects of ferulic and sinapic acids in pre-cultures were totally opposite to those of the submerged culture, directing the carbon distribution into vanillin formation. In surface culture, more than 30% of ferulic acid can be used as carbon source for other metabolic processes, such as ATP regeneration. In this way, the intracellular ATP concentration remained constant during the biotransformation process by surface culture (100 μg ATP/mg protein), demonstrating a high energetic state, which can maintain active the non-oxidative deacetylation pathway. In contrast, in submerged culture, it decreased 3.15-fold at the end of the biotransformation compared with the initial content, showing a low energetic state, while the NAD+/NADH ratio (23.15) increased 1.81-fold. It seems that in submerged culture, low energetic and high oxidative states are the physiological conditions that can redirect the ferulic catabolism into β-oxidative pathway and/or vanillin oxidation to produce vanillic acid.




Similar content being viewed by others
References
Sutherland, J. B., Crawford, D. L., & Pometto III, A. L. (1983). Metabolism of cinnamic, p-coumaric, and ferulic acids by Streptomyces setonii. Canadian Journal of Microbiology, 29(10), 1253–1257.
Brown, M. E., Walker, M. C., Nakashige, T. G., Iavarone, A. T., & Chang, M. C. (2011). Discovery and characterization of heme enzymes from unsequenced bacteria: application to microbial lignin degradation. Journal of the American Chemical Society, 133(45), 18006–18009.
Kaur, B., & Chakraborty, D. (2013). Biotechnological and molecular approaches for vanillin production: a review. Applied Biochemistry and Biotechnology, 169(4), 1353–1372.
Asaff, A., & Reyes, Y. (2018) Method and system for the integral treatment of wastewater from the maize industry. US Patent 10,011,509 B2.
Meyer, F., Netzer, J., Meinert, C., Voigt, B., Riedel, K., & Steinbüchel, A. (2018). A proteomic analysis of ferulic acid metabolism in Amycolatopsis sp. ATCC 39116. Applied Microbiology and Biotechnology, 102(14), 6119–6142.
Gallage, N. J., & Møller, B. L. (2015). Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Molecular Plant, 8(1), 40–57.
Fleige, C., Hansen, G., Kroll, J., & Steinbüchel, A. (2013). Investigation of the Amycolatopsis sp. strain ATCC 39116 vanillin dehydrogenase and its impact on the biotechnical production of vanillin. Applied and Environmental Microbiology, 79(1), 81–90.
Cai, R., Yuan, Y., Wang, Z., Guo, C., Liu, B., Liu, L., & Yue, T. (2015). Precursors and metabolic pathway for guaiacol production by Alicyclobacillus acidoterrestris. International Journal of Food Microbiology, 214, 48–53.
Johnson, C. W., & Beckham, G. T. (2015). Aromatic catabolic pathway selection for optimal production of pyruvate and lactate from lignin. Metabolic Engineering, 28, 240–247.
Kallscheuer, N., Vogt, M., Kappelmann, J., Krumbach, K., Noack, S., Bott, M., & Marienhagen, J. (2016). Identification of the phd gene cluster responsible for phenylpropanoid utilization in Corynebacterium glutamicum. Applied Microbiology and Biotechnology, 100(4), 1871–1881.
Kasai, D., Kamimura, N., Tani, K., Umeda, S., Abe, T., Fukuda, M., & Masai, E. (2012). Characterization of FerC, a MarR-type transcriptional regulator, involved in transcriptional regulation of the ferulate catabolic operon in Sphingobium sp. strain SYK-6. FEMS Microbiology Letters, 332, 68–75.
Ma, X. K., & Daugulis, A. J. (2014). Effect of bioconversion conditions on vanillin production by Amycolatopsis sp. ATCC 39116 through an analysis of competing by-product formation. Bioprocess and Biosystems Engineering, 37, 891–899.
Asaff, A., De La Torre, M., Mir, A. B., & Ochoa, R. M. M. (2011) Process of production of vanillin with immobilized microorganisms. U.S. patent 2011/0065156A1.
González-Segura, L., Velasco-García, R., & Muñoz-Clares, R. A. (2002). Modulation of the reactivity of the essential cysteine residue of betaine aldehyde dehydrogenase from Pseudomonas aeruginosa. The Biochemical Journal, 361(3), 577–585.
AOAC. (2000). Official methods of analysis. Official method 986.35 (16th ed.). Gaithersburg: Association of Official Analytical Chemists.
Preciado-Saldaña, A. M., Abraham Domínguez-Avila, J., Fernando Ayala-Zavala, J., Villegas-Ochoa, M. A., Sáyago-Ayerdi, S. G., Wall-Medrano, A., & González-Aguilar, G. A. (2019). Formulation and characterization of an optimized functional beverage from hibiscus (Hibiscus sabdariffa L.) and green tea (Camellia sinensis L.). Food Science and Technology International, 25, 547–561.
Ou, B., Hampsch-Woodill, M., & Prior, R. L. (2001). Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. Journal of Agricultural and Food Chemistry, 49(10), 4619–4626.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Fleige, C., Meyer, F., & Steinbüchel, A. (2016). Metabolic engineering of the actinomycete Amycolatopsis sp. strain ATCC 39116 towards enhanced production of natural vanillin. Applied and Environmental Microbiology, 82(11), 3410–3419.
Martínez-Cuesta, M., Payne, J., Hanniffy, S. B., Gasson, M. J., & Narbad, A. (2005). Functional analysis of the vanillin pathway in a vdh-negative mutant strain of Pseudomonas fluorescens AN103. Enzyme and Microbial Technology, 37(1), 131–138.
Civolani, C., Barghini, P., Roncetti, A. R., Ruzzi, M., & Schiesser, A. (2000). Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13. Applied and Environmental Microbiology, 66(6), 2311–2317.
Achterholt, S., Priefert, H., & Steinbüchel, A. (2000). Identification of Amycolatopsis sp. strain HR167 genes, involved in the bioconversion of ferulic acid to vanillin. Applied Microbiology and Biotechnology, 54(6), 799–807.
Crawford, R. L., & Olson, P. P. (1978). Microbial catabolism of vanillate: decarboxylation to guaiacol. Applied and Environmental Microbiology, 36(4), 539–543.
Chow, K. T., Pope, M. K., & Davies, J. (1999). Characterization of a vanillic acid non-oxidative decarboxylation gene cluster from Streptomyces sp. D7. Microbiology., 145(9), 2393–2403.
Aguilar, C. N., Augur, C., Favela-Torres, E., & Viniegra-González, G. (2001). Induction and repression patterns of fungal tannase in solid-state and submerged cultures. Process Biochemistry, 36(6), 565–570.
Renovato, J., Gutiérrez-Sánchez, G., Rodríguez-Durán, L. V., Bergman, C., Rodríguez, R., & Aguilar, C. N. (2011). Differential properties of Aspergillus niger tannase produced under solid-state and submerged fermentations. Applied Biochemistry and Biotechnology, 165(1), 382–395.
Hernández-Domínguez, E. M., Rios-Latorre, R. A., Álvarez-Cervantes, J., Loera-Corral, O., Román-Gutiérrez, A. D., Díaz-Godínez, G., & Mercado-Flores, Y. (2014). Xylanases, cellulases, and acid protease produced by Stenocarpella maydis grown in solid-state and submerged fermentation. BioResources, 9, 2341–2358.
Barrios-González, J., Baños, J. G., Covarrubias, A. A., & Garay-Arroyo, A. (2008). Lovastatin biosynthetic genes of Aspergillus terreus are expressed differentially in solid-state and in liquid submerged fermentation. Applied Microbiology and Biotechnology, 79(2), 179–186.
te Biesebeke, R., van Biezen, N., De Vos, W. M., Van Den Hondel, C. A. M. J. J., & Punt, P. J. (2005). Different control mechanisms regulate glucoamylase and protease gene transcription in Aspergillus oryzae in solid-state and submerged fermentation. Applied Microbiology and Biotechnology, 67(1), 75–82.
Otani, H., Stogios, P. J., Xu, X., Nocek, B., Li, S. N., Savchenko, A., & Eltis, L. D. (2015). The activity of CouR, a MarR family transcriptional regulator, is modulated through a novel molecular mechanism. Nucleic Acids Research, 44, 595–607.
Davis, J. R., Goodwin, L. A., Woyke, T., Teshima, H., Bruce, D., Detter, C., & Nolan, M. (2012). Genome sequence of Amycolatopsis sp. strain ATCC 39116, a plant biomass-degrading actinomycete. Journal of Bacteriology, 194(9), 2396–2397.
Wilkinson, S. P., & Grove, A. (2006). Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Current Issues in Molecular Biology, 8, 51–62.
Parke, D., & Ornston, L. N. (2003). Hydroxycinnamate (hca) catabolic genes from Acinetobacter sp. strain ADP1 are repressed by HcaR and are induced by hydroxycinnamoyl-coenzyme a thioesters. Applied and Environmental Microbiology, 69(9), 5398–5409.
Grove, A. (2013). MarR family transcription factors. Current Biology, 23(4), R142–R143.
Aoki, R., Takeda, T., Omata, T., Ihara, K., & Fujita, Y. (2012). MarR-type transcriptional regulator ChlR activates expression of tetrapyrrole biosynthesis genes in response to low-oxygen conditions in cyanobacteria. The Journal of Biological Chemistry, 287(16), 13500–13507.
Fuangthong, M., & Helman, J. D. (2002). The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proceedings of the National Academy of Sciences of the United States of America, 99(10), 6690–6695.
Panmanee, W., Vattanaviboon, P., Poole, L. B., & Mongkolsuk, S. (2006). Novel organic hydroperoxide-sensing and responding mechanisms for OhrR, a major bacterial sensor and regulator of organic hydroperoxide stress. Journal of Bacteriology, 188(4), 1389–1395.
Miranda, R. U., Gómez-Quiroz, L. E., Mejía, A., & Barrios-González, J. (2013). Oxidative state in idiophase links reactive oxygen species (ROS) and lovastatin biosynthesis: differences and similarities in submerged-and solid-state fermentations. Fungal Biology, 117(2), 85–93.
Miranda, R. U., Gómez-Quiroz, L. E., Mendoza, M., Pérez-Sánchez, A., Fierro, F., & Barrios-González, J. (2014). Reactive oxygen species regulate lovastatin biosynthesis in Aspergillus terreus during submerged and solid-state fermentations. Fungal Biology, 118(12), 979–989.
Perera, I. C., & Grove, A. (2010). Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. Journal of Molecular Cell Biology, 2(5), 243–254.
Otani, H., Lee, Y. E., Casabon, I., & Eltis, L. D. (2014). Characterization of p-hydroxycinnamate catabolism in a soil Actinobacterium. Journal of Bacteriology, 196(24), 4293–4303.
Mallinson, S. J., Machovina, M. M., Silveira, R. L., Garcia-Borràs, M., Gallup, N., Johnson, C. W., & Houk, K. N. (2018). A promiscuous cytochrome P450 aromatic O-demethylase for lignin bioconversion. Nature Communications, 9, 1–12.
Romero-Gómez, S. J., Augur, C., & Viniegra-González, G. (2000). Invertase production by Aspergillus niger in submerged and solid-state fermentation. Biotechnology Letters, 22(15), 1255–1258.
Asaff, A., Cerda-García-Rojas, C. M., Viniegra-González, G., & de la Torre, M. (2006). Carbon distribution and redirection of metabolism in Paecilomyces fumosoroseus during solid-state and liquid fermentations. Process Biochemistry, 41(6), 1303–1310.
Lima-Pérez, J., López-Pérez, M., Viniegra-González, G., & Loera, O. (2019). Solid-state fermentation of Bacillus thuringiensis var kurstaki HD-73 maintains higher biomass and spore yields as compared to submerged fermentation using the same media. Bioprocess and Biosystems Engineering, 42, 1–9.
Garcia-Ochoa, F., Gomez, E., Santos, V. E., & Merchuk, J. C. (2010). Oxygen uptake rate in microbial processes: an overview. Biochemical Engineering Journal, 49(3), 289–307.
Jouhten, P., Rintala, E., Huuskonen, A., Tamminen, A., Toivari, M., Wiebe, M., & Maaheimo, H. (2008). Oxygen dependence of metabolic fluxes and energy generation of Saccharomyces cerevisiae CEN. PK113-1A. BMC Systems Biology, 2(1), 60.
Fuchs, G., Boll, M., & Heider, J. (2011). Microbial degradation of aromatic compounds—From one strategy to four. Nature Reviews. Microbiology, 9(11), 803–816.
Akram, M. (2014). Citric acid cycle and role of its intermediates in metabolism. Cell Biochemistry and Biophysics, 68(3), 475–478.
De Graef, M. R., Alexeeva, S., Snoep, J. L., & de Mattos, M. J. T. (1999). The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli. Journal of Bacteriology, 181(8), 2351–2357.
Friedrich, T. (1998). The NADH: ubiquinone oxidoreductase (complex I) from Escherichia coli. Biochimica et Biophysica Acta - Bioenergetics, 1364(2), 134–146.
Du, C., Yan, H., Zhang, Y., Li, Y., & Cao, Z. A. (2006). Use of oxidoreduction potential as an indicator to regulate 1, 3-propanediol fermentation by Klebsiella pneumoniae. Applied Microbiology and Biotechnology, 69(5), 554–563.
Simon, O., Klaiber, I., Huber, A., & Pfannstiel, J. (2014). Comprehensive proteome analysis of the response of Pseudomonas putida KT2440 to the flavor compound vanillin. Journal of Proteomics, 109, 212–227.
Coitinho, J. B., Pereira, M. S., Costa, D. M., Guimarães, S. L., Araújo, S. S., Hengge, A. C., & Nagem, R. A. (2016). Structural and kinetic properties of the aldehyde dehydrogenase NahF, a broad substrate specificity enzyme for aldehyde oxidation. Biochemistry., 55(38), 5453–5463.
Karala, A. R., & Ruddock, L. W. (2007). Does s-methyl methanethiosulfonate trap the thiol–disulfide state of proteins? Antioxidants & Redox Signaling, 9(4), 527–531.
Velasco-García, R., Zaldívar-Machorro, V. J., Mújica-Jiménez, C., González-Segura, L., & Muñoz-Clares, R. A. (2006). Disulfiram irreversibly aggregates betaine aldehyde dehydrogenase—A potential target for antimicrobial agents against Pseudomonas aeruginosa. Biochemical and Biophysical Research Communications, 341(2), 408–415.
Kim, J. J. P., & Miura, R. (2004). Acyl-CoA dehydrogenases and acyl-CoA oxidases: structural basis for mechanistic similarities and differences. European Journal of Biochemistry, 271(3), 483–493.
Amari, D. T., Marques, C. N., & Davies, D. G. (2013). The putative enoyl-CoA hydratase DspI is required for production of the Pseudomonas aeruginosa biofilm bispersion autoinducer, cis-2-decenoic acid. Journal of Bacteriology, 95, 4600–4610.
Acknowledgments
V. Contreras-Jácquez is thankful to the (CONACYT) scholarship awarded and to Mónica A. Villegas-Ochoa for her helpful assistance.
Funding
This work is financially supported by the Mexican Council of Science and Technology (CONACyT), grant no. 2017-01-6267.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Victor Contreras-Jácquez. The first draft of the manuscript was written by Victor Contreras-Jácquez, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 556 kb)
Rights and permissions
About this article
Cite this article
Contreras-Jácquez, V., Rodríguez-González, J., Mateos-Díaz, J.C. et al. Differential Activation of Ferulic Acid Catabolic Pathways of Amycolatopsis sp. ATCC 39116 in Submerged and Surface Cultures. Appl Biochem Biotechnol 192, 494–516 (2020). https://doi.org/10.1007/s12010-020-03336-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03336-4