Abstract
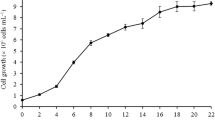
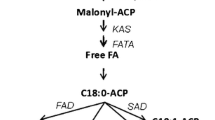
Abscisic acid (ABA) has been known to exist in a microalgal system and serves as one of the chemical stimuli in various biological pathways. Nonetheless, the involvement of ABA in fatty acid biosynthesis, particularly at the transcription level in microalgae is poorly understood. The objective of this study was to determine the effects of exogenous ABA on growth, total oil content, fatty acid composition, and the expression level of beta ketoacyl-ACP synthase I (KAS I) and omega-3 fatty acid desaturase (ω-3 FAD) genes in Chlorella vulgaris UMT-M1. ABA was applied to early stationary C. vulgaris cultures at concentrations of 0, 10, 20, and 80 μM for 48 h. The results showed that ABA significantly increased biomass production and total oil content. The increment of palmitic (C16:0) and stearic (C18:0) acids was coupled by decrement in linoleic (C18:2) and α-linolenic (C18:3n3) acids. Both KAS I and ω-3 FAD gene expression were downregulated, which was negatively correlated to saturated fatty acid (SFAs), but positively correlated to polyunsaturated fatty acid (PUFA) accumulations. Further analysis of both KAS I and ω-3 FAD promoters revealed the presence of multiple ABA-responsive elements (ABREs) in addition to other phytohormone-responsive elements. However, the role of these phytohormone-responsive elements in regulating KAS I and ω-3 FAD gene expression still remains elusive. This revelation might suggest that phytohormone-responsive gene regulation in C. vulgaris and microalgae as a whole might diverge from higher plants which deserve further scientific research to elucidate its functional roles.




Similar content being viewed by others
References
Lu, Y., & Xu, J. (2015). Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends in Plant Science, 20(5), 273–282.
Contreras-Pool, P. Y., Peraza-Echeverria, S., Ku-Gonzalez, A. F., & Herrera-Valencia, V. A. (2016). The phytohormone abscisic acid increases triacylglycerol content in the green microalga Chlorella saccharophila (Chlorophyta). Algae, 31(3), 267–276.
Du, H., Ahmed, F., Lin, B., Li, Z., Huang, Y., Sun, G., Ding, H., Wang, C., Meng, C., & Gao, Z. (2017). The effects of plant growth regulators on cell growth, protein, carotenoid, PUFAs and lipid production of Chlorella pyrenoidosa ZF strain. Energies, 10(1), 1696.
Wu, G., Gao, Z., Du, H., & Meng, C. (2018). The effects of abscisic acid, salicylic acid and jasmonic acid on lipid accumulation in two freshwater Chlorella strains. The Journal of General and Applied Microbiology, 64(1), 42–49.
Liu, T., Liu, F., Wang, C., Wang, Z., & Li, Y. (2017). The boosted biomass and lipid accumulation in Chlorella vulgaris by supplementation of synthetic phytohromone analogs. Bioresource Technology, 232, 44–52.
Jusoh, M., Loh, S. H., Chuah, T. S., Aziz, A., & Cha, T. S. (2015). Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Research, 9, 14–20.
Kozlova, T. A., Hardy, B. P., Krishna, P., & Levin, D. B. (2017). Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Research, 27, 325–334.
Kaleem, F., Shabir, G., Aslam, K., Rasul, S., Manzoor, H., Shah, S. M., & Khan, A. R. (2018). An overview of the genetics of plant response to salt stress: present status and the way forward. Applied Biochemistry and Biotechnology, 186(2), 306–334.
Sulochana, S. B., & Arumugam, M. (2016). Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresource Technology, 213, 198–203.
Ohlrogge, J. B., & Browse, J. (1995). Lipid biosynthesis. Plant Cell, 7(7), 957–970.
Salas, J. J., Sánchez, J., Ramli, U. S., Manaf, A. M., Williams, M., & Harwood, J. L. (2000). Biochemistry of lipid metabolism in olive and other oil fruits. Progress in Lipid Research, 39(2), 151–180.
Liu, J., Huang, J., Sun, Z., Zhong, Y., Jiang, Y., & Chen, F. (2011). Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresource Technology, 102(1), 106–110.
Jusoh, M., Loh, S. H., Chuah, T. S., Aziz, A., & Cha, T. S. (2015). Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry, 111, 65–71.
Cha, T. S., Chen, J. W., Goh, E. G., Aziz, A., & Loh, S. H. (2011). Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresource Technology, 102(22), 10633–10640.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408.
Lin, J., Jin, Y., Wang, J., & Tang, K. (2008). Cloning and analysis of the 5′ and 3′ flanking regions of the Crinum asiaticum agglutinin gene by genomic walking. African Journal of Biotechnology, 7, 3582–3586.
Lescot, M., Déhais, P., Thijs, G., Marchal, K., Moreau, Y., Van de Peer, Y., Rouzé, P., & Rombauts, S. (2002). PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequence. Nucleic Acids Research, 30(1), 325–327.
Huang, G. T., Ma, S. L., Bai, L. P., Zhang, L., Ma, H., Jia, P., Liu, J., Zhong, M., & Guo, Z. F. (2012). Signal transduction during cold, salt, and drought stresses in plants. Molecular Biology Reports, 39(2), 969–987.
Kobayashi, M., Hirai, N., Kurimura, Y., Ohigashi, H., & Tsuji, Y. (1997). Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis. Plant Growth Regulator, 22(2), 79–85.
Lu, Y., Tarkowská, D., Turečková, V., Luo, T., Xin, Y., Li, J., Wang, Q., Jiao, N., Strnad, M., & Xu, J. (2014). Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. The Plant Journal, 80(1), 52–68.
Park, W. K., Yoo, G., Moon, M., Kim, C. W., Choi, Y. E., & Yang, J. W. (2013). Phytohormone supplementation significantly increases growth of Chlamydomonas reinhardtii cultivated for biodiesel production. Applied Biochemistry and Biotechnology, 171(5), 1128–1142.
Nguyen, Q. T., Kisiala, A., Andreas, P., Neil Emery, R. J., & Narine, S. (2016). Soybean seed development: fatty acid and phytohormone metabolism and their interactions. Current Genomics, 17(3), 241–260.
Ra, C. H., Kang, C. H., Jung, J. H., Jeong, G. T., & Kim, S. K. (2016). Effects of light-emitting diodes (LEDs) on the accumulation of lipid content using a two-phase culture process with three microalgae. Bioresource Technology, 212, 254–261.
Nagappan, S., Devendran, S., Tsai, P. C., & Dahms, H. U. (2019). Potential of two-stage cultivationin microalgae biofuel production. Fuel, 252, 339–349.
Feng, Y., Li, C., & Zhang, D. (2011). Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresource Technology, 102(1), 101–105.
Lin, B., Ahmed, F., Du, H., Li, Z., Yan, Y., Huang, Y., Cui, M., Yin, Y., Li, B., Wang, M., Meng, C., & Gao, Z. (2018). Plant growth regulators promote lipid and carotenoid accumulation in Chlorella vulgaris. Journal of Applied Phycology, 30(3), 1549–1561.
Pacheco-Moises, F., Valencia-Turcotte, L., Altuzar-Martinez, M., & Rodriguez-Sotres, R. (1997). Regulation of acyltransferase activity in immature maize embryos by abscisic acid and the osmotic environment. Plant Physiology, 114(3), 1095–1101.
Jusoh, M., Loh, S. H., Aziz, A., & Cha, T. S. (2019). Gibberellin promotes cell growth and induces changes in fatty acid biosynthesis and upregulates fatty acid biosynthesis genes in Chlorella vulgaris UMT-M1. Applied Biochemistry and Biotechnology, 188(2), 450–459.
Knothe, G., Matheaus, A. C., & Ryan III, T. W. (2003). Cetane number of branched and straight-chain fatty esters determined in an ignition quality tester. Fuel, 82, 971–975.
Ramos, M. J., Fernández, C. M., Casas, A., Rodríguez, L., & Pérez, A. (2009). Influence of fatty acid composition of raw materials on biodiesel properties. Bioresource Technology, 100(1), 261–268.
Kong, Y., Chen, S., Yang, Y., & An, C. (2013). ABA-insensitive (ABI) 4 and ABI5 synergistically regulate DGAT1 expression in Arabidopsis seedlings under stress. FEBS Letters, 587(18), 3076–3082.
Jako, C., Kumar, A., Wei, Y., Zou, J., Barton, D. L., Giblin, E. M., Covello, P. S., & Taylor, D. C. (2001). Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiology, 126(2), 861–874.
Beacham, T. A., & Ali, S. T. (2016). Growth dependent silencing and resetting of DGA1 transgene in Nannochloropsis salina. Algal Research, 14, 65–71.
Liang, M. H., & Jiang, J. G. (2013). Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Progress in Lipid Research, 52(4), 395–408.
Lei, A. P., Chen, H., Shen, G. M., Hu, Z. L., Chen, L., & Wang, J. X. (2012). Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnology for Biofuels, 5, 18.
Norashikin, M. N., Loh, S. H., Aziz, A., & Cha, T. S. (2018). Metabolic engineering of fatty acid biosynthesis in Chlorella vulgaris using an endogenous omega-3 fatty acid desaturase gene with its promoter. Algal Research, 31, 262–275.
Holbrook, L. A., Magus, J. R., & Taylor, D. C. (1992). Abscisic acid induction of elongase activity, biosynthesis and accumulation of very long chain monounsaturated fatty acids and oil body proteins in microspore-derived embryos of Brassica napus L. cv Reston. Plant Science, 84(1), 99–115.
Kunst, L., Taylor, D. C., & Underhill, E. W. (1992). Fatty acid elongation in developing seeds of Arabidopsis thaliana affecting wax biosynthesis in Arabidopsis thaliana. Plant Physiology and Biochemistry, 30(4), 425–434.
Henry, G. E., Momin, R. A., Nair, M. G., & Dewitt, D. L. (2002). Antioxidant and cyclooxygenase activities of fatty acids found in food. Journal of Agricultural and Food Chemistry, 50(8), 2231–2234.
Lee, J. H., Kim, Y. G., Park, J. G., & Lee, J. (2017). Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control, 80, 74–82.
Fujita, Y., Fujita, M., Shinozaki, K., & Yamaguchi-Shinozaki, K. (2011). ABA-mediated transcriptional regulation in response to osmotic stress in plants. Journal of Plant Research, 124(4), 509–525.
Kim, J. A., Bhatnagar, N., Kwon, S. J., Min, M. K., Moon, S. J., Yoon, I. S., Kwon, T. R., Kim, S. T., & Kim, B. G. (2018). Transcriptome analisis of ABA/JA-dual responsive genes in rice shoot and root. Current Genomics, 19(1), 4–11.
Verma, V., Ravindran, P., & Kumar, P. P. (2016). Plant hormone-mediated regulation of stress responses. BMC Plant Biology, 16, 86.
Gómez, J. L., Riaño-Pachón, D. M., Dreyer, I., Mayer, J. E., & Mueller-Roeber, B. (2007). Genome-wide analysis of ABA-responsive elements ABRE and CE3 reveals divergent patterns in Arabidopsis and rice. BMC Genomics, 8, 260.
Nakashima, K., Fujita, Y., Katsura, K., Maruyama, K., Narusaka, Y., Seki, M., Shinozaki, K., & Yamaguchi-Shinozaki, K. (2006). Transcriptional regulation of ABI3- and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Molecular Biology, 60(1), 51–68.
Kashyap, P., & Deswal, R. (2019). Two ICE isoforms showing differential transcriptional regulation by cold and hormones participate in Brassica juncea cold stress signaling. Gene, 695, 32–41.
Song, N., Xu, Z., Wang, J., Qin, Q., Jiang, H., Si, W., & Li, X. (2018). Genome-wide analysis of maize CONSTANS-LIKE gene family and expression profiling under light/dark and abscisic acid treatment. Gene, 673, 1–11.
Xiao, G., Zhang, Z. Q., Yin, C. F., Liu, R. Y., Wu, X. M., Tan, T. L., Chen, S. Y., Lu, C. M., & Guan, C. Y. (2014). Characterization of the promoter and 5’-UTR intron of oleic acid desaturase (FAD2) gene in Brassica napus. Gene, 545(1), 45–55.
Zhang, W., Ruan, J., David Ho, T., You, Y., Yu, T., & Quatrano, R. S. (2005). cis-Regulatory element based targeted gene finding: genome-wide identification of abscisic acid- and abiotic stress-responsive genes in Arabidopsis thaliana. Bioinformatics, 21(14), 3074–3081.
Kim, J. S., Mizoi, J., Yoshida, T., Fujita, Y., Nakajima, J., Ohori, T., Tokada, D., Nakashima, K., Hitayama, T., Shinozaki, K., & Kazuko, Y. S. (2011). An ABRE promoter seqeunce is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible gene in Arabidopsis. Plant and Cell Physiology, 52(12), 2136–2146.
Funding
This research project was funded under a Science Fund (Project No: 02-01-12-SF0089) from the Ministry of Science, Technology and Innovation (MOSTI), Malaysia.
Author information
Authors and Affiliations
Contributions
TSC, RN, MNN, SHL, and AA conceived and designed the research; RN and MNN conducted the experiments. TSC, SHL, and AA analyzed and interpreted the data. TSC and AA wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Consent for Publication
All authors approved the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Norlina, R., Norashikin, M.N., Loh, S.H. et al. Exogenous Abscisic Acid Supplementation at Early Stationary Growth Phase Triggers Changes in the Regulation of Fatty Acid Biosynthesis in Chlorella vulgaris UMT-M1. Appl Biochem Biotechnol 191, 1653–1669 (2020). https://doi.org/10.1007/s12010-020-03312-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03312-y