Abstract
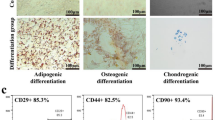
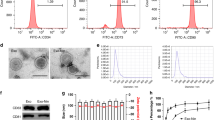
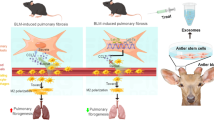
Pulmonary fibrosis (PF) is a progressive and irreversible lung disease, characterized by poor prognosis with limited treatment options. Mesenchymal stem cells (MSCs) are multi-potent cells having the ability to self-renew and differentiate into multiple tissues, thus considered a novel treatment option. The present study aimed to investigate the possible antifibrotic effect of undifferentiated adipose tissue–derived mesenchymal stem cell (AD-MSC) therapy on PF experimentally induced in rats using amiodarone (AMD). AMD (30 mg/kg) was given orally, once daily for 12 consecutive weeks to induce lung fibrosis. Following the confirmation of lung damage with histopathological examination, AD-MSCs (2 × 106 and 4 × 106 undifferentiated MSCs) were injected once intravenously, followed by 2 months for treatment. AMD induced focal fibroblastic cells proliferation in the peribronchiolar tissue, as well as in between the collapsed emphysematous alveoli. Also, AMD significantly increased serum and lung homogenate fibroblast growth factor-7 (FGF7), Clara cell protein-16 (CC16), and cytokeratin -19 (CK19) levels, as well as the expression of both iNOS and NFкB in the lung alveoli. Moreover, AMD caused excessive collagen deposition and increased alpha smooth muscle actin (α-SMA) expression. All findings significantly regressed on stem cell therapy in both doses, with superior effect of the high dose, providing evidence that adipose tissue–derived MSCs could be a promising approach for the treatment of PF.

Graphical Abstract





Similar content being viewed by others
References
Chioma, O. S., & Drake, W. P. (2017). Role of microbial agents in pulmonary fibrosis. The Yale Journal of Biology and Medicine, 90(2), 219–227.
Reddy, M., Fonseca, L., Gowda, S., Chougule, B., Hari, A., & Totey, S. (2016). Human adipose-derived mesenchymal stem cells attenuate early stage of bleomycin induced pulmonary fibrosis: comparison with pirfenidone. International Journal of Stem Cells, 9(2), 192–206.
Zhao, D., Hou, L., Pan, M., Hua, J., Wang, Z., He, J., & Hu, H. (2018). Inhibitory effect and mechanism of mesenchymal stem cells cultured in 3D system on hepatoma cells HepG2. Applied Biochemistry and Biotechnology, 184(1), 212–227.
Cai, C., Hou, L., Zhang, J., Zhao, D., Wang, Z., Hu, H., He, J., Guan, W., & Ma, Y. (2018). Author correction: the inhibitory effect of mesenchymal stem cells with rAd-NK4 on liver cancer. Applied Biochemistry and Biotechnology, 185(1), 357.
Fikry, E. M., Safar, M. M., Hasan, W. A., Fawzy, H. M., & el-Denshary, E. E. (2015). Bone marrow and adipose-derived mesenchymal stem cells alleviate methotrexate-induced pulmonary fibrosis in rat: comparison with dexamethasone. Journal of Biochemical and Molecular Toxicology, 29(7), 321–329.
Al-Shammari, B., et al. (2016). A mechanistic study on the amiodarone-induced pulmonary toxicity. Oxidative Medicine and Cellular Longevity, 2016.
Raeder, E. A., Podrid, P. J., & Lown, B. (1985). Side effects and complications of amiodarone therapy. American Heart Journal, 109(5), 975–983.
Mahavadi, P., Henneke, I., Ruppert, C., Knudsen, L., Venkatesan, S., Liebisch, G., Chambers, R. C., Ochs, M., Schmitz, G., Vancheri, C., Seeger, W., Korfei, M., & Guenther, A. (2014). Altered surfactant homeostasis and alveolar epithelial cell stress in amiodarone-induced lung fibrosis. Toxicological Sciences, 142(1), 285–297.
Baumann, H., Fichtenkamm, P., Schneider, T., Biscoping, J., & Henrich, M. (2017). Rapid onset of amiodarone induced pulmonary toxicity after lung lobe resection–a case report and review of recent literature. Annals of Medicine and Surgery, 21, 53–57.
Piccini, J. P., Berger, J. S., & O’Connor, C. M. (2009). Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. European Heart Journal, 30(10), 1245–1253.
Leeder, R. G., Brien, J. F., & Massey, T. E. (1994). Investigation of the role of oxidative stress in amiodarone-induced pulmonary toxicity in the hamster. Canadian Journal of Physiology and Pharmacology, 72(6), 613–621.
Schwaiblmair, M., Berghaus, T., Haeckel, T., Wagner, T., & von Scheidt, W. (2010). Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clinical Research in Cardiology, 99(11), 693–700.
Reasor, M. J., & Kacew, S. (1996). An evaluation of possible mechanisms underlying amiodarone-induced pulmonary toxicity. Proceedings of the Society for Experimental Biology and Medicine, 212(4), 297–304.
Wolkove, N., & Baltzan, M. (2009). Amiodarone pulmonary toxicity. Canadian Respiratory Journal, 16(2), 43–48.
Martin, W., & Rosenow, E. (1988). Amiodarone pulmonary toxicity: recognition and pathogenesis (Part 2). Chest, 93(6), 1242–1248.
Tomiyama, K., Murase, N., Stolz, D. B., Toyokawa, H., O’Donnell, D. R., Smith, D. M., Dudas, J. R., Rubin, J. P., & Marra, K. G. (2008). Characterization of transplanted green fluorescent protein+ bone marrow cells into adipose tissue. Stem Cells, 26(2), 330–338.
Alhadlaq, A., & Mao, J. J. (2004). Mesenchymal stem cells: isolation and therapeutics. Stem Cells and Development, 13(4), 436–448.
Woodbury, D., Schwarz, E. J., Prockop, D. J., & Black, I. B. (2000). Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of Neuroscience Research, 61(4), 364–370.
Zidan, R. A. (2011). Effect of long-term administration of amiodarone on rat lung and the possible protective role of vitamin E: a histological and immunohistochemical study. Egyptian Journal of Histology, 34(1), 117–128.
Zaglool, S. S., Zickri, M. B., Abd el Aziz, D. H., Mabrouk, D., & Metwally, H. G. (2011). Effect of stem cell therapy on amiodarone induced fibrosing interstitial lung disease in albino rat. International Journal of Stem Cells, 4(2), 133–142.
Bancroft, J. D., & Gamble, M. (2008). Theory and practice of histological techniques. Elsevier Health Sciences.
Buchwalow, I. B., & Böcker, W. (2010). Immunohistochemistry. Basics and Methods, 1, 1–149.
Geiger, S., Hirsch, D., & Hermann, F. G. (2017). Cell therapy for lung disease. European Respiratory Review, 26(144), 170044.
Tata, P. R., & Rajagopal, J. (2017). Plasticity in the lung: making and breaking cell identity. Development, 144(5), 755–766.
Uhal, B. D., Wang, R., Laukka, J., Zhuang, J., Soledad-Conrad, V., & Filippatos, G. (2003). Inhibition of amiodarone-induced lung fibrosis but not alveolitis by angiotensin system antagonists. Pharmacology & Toxicology, 92(2), 81–87.
Castro-Manrreza, M. E., & Montesinos, J. J. (2015). Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. Journal of Immunology Research, 2015.
Nurkovic, J., Dolicanin, Z., Mustafic, F., Mujanovic, R., Memic, M., Grbovic, V., Skevin, A. J., & Nurkovic, S. (2016). Mesenchymal stem cells in regenerative rehabilitation. Journal of Physical Therapy Science, 28(6), 1943–1948.
Punithavathi, D., Venkatesan, N., & Babu, M. (2003). Protective effects of curcumin against amiodarone-induced pulmonary fibrosis in rats. British Journal of Pharmacology, 139(7), 1342–1350.
Moodley, Y., Atienza, D., Manuelpillai, U., Samuel, C. S., Tchongue, J., Ilancheran, S., Boyd, R., & Trounson, A. (2009). Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. The American Journal of Pathology, 175(1), 303–313.
Nagata, N., Suematsu, R., Yoshii, C., Miyazaki, H., Sueishi, K., & Kido, M. (1997). Characterization of amiodarone pneumonitis as related to inflammatory cells and surfactant apoprotein. Chest, 112(4), 1068–1074.
Cinar, R., et al. (2017). Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight, 2(8).
Kalayarasan, S., Sriram, N., & Sudhandiran, G. (2008). Diallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-kappaB, TNF-alpha and IL-1beta. Life Sciences, 82(23–24), 1142–1153.
Choi, H. J., Park, J. H., Lee, B. H., Chee, H. Y., Lee, K. B., & Oh, S. M. (2014). Suppression of NF-kappaB by dieckol extracted from Ecklonia cava negatively regulates LPS induction of inducible nitric oxide synthase gene. Applied Biochemistry and Biotechnology, 173(4), 957–967.
Naura, A. S., et al. (2010). Requirement for inducible nitric oxide synthase in chronic allergen exposure-induced pulmonary fibrosis but not inflammation. Journal of Immunology, 185(5), 3076–3085.
Pullamsetti, S. S., et al. (2011). The role of dimethylarginine dimethylaminohydrolase in idiopathic pulmonary fibrosis. Science Translational Medicine, 3(87), 87ra53.
Alvira, C. M. (2014). Nuclear factor-kappa-B signaling in lung development and disease: One pathway, numerous functions. Birth Defects Research. Part A, Clinical and Molecular Teratology, 100(3), 202–216.
Pahl, H. L. (1999). Activators and target genes of Rel/NF-κB transcription factors. Oncogene, 18(49), 6853–6866.
Gregersen, P. K., Amos, C. I., Lee, A. T., Lu, Y., Remmers, E. F., Kastner, D. L., Seldin, M. F., Criswell, L. A., Plenge, R. M., Holers, V. M., Mikuls, T. R., Sokka, T., Moreland, L. W., Bridges SL Jr, Xie, G., Begovich, A. B., & Siminovitch, K. A. (2009). REL, encoding a member of the NF-κB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nature Genetics, 41(7), 820–823.
Hollenbach, E., Neumann, M., Vieth, M., Roessner, A., Malfertheiner, P., & Naumann, M. (2004). Inhibition of p38 MAP kinase-and RICK/NF-κB-signaling suppresses inflammatory bowel disease. The FASEB Journal, 18(13), 1550–1552.
Tang, X., et al. (2006). Nuclear factor-κB (nf-κB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer: Interdisciplinary International Journal of the American Cancer Society, 107(11), 2637–2646.
Abdelmawgoud, H., & Saleh, A. (2018). Anti-inflammatory and antioxidant effects of mesenchymal and hematopoietic stem cells in a rheumatoid arthritis rat model. Advances in Clinical and Experimental Medicine: Official Organ Wroclaw Medical University, 27(7), 873–880.
Regulski, M. J. (2017). Mesenchymal stem cells:" guardians of inflammation". Wounds: a Compendium of Clinical Research and Practice, 29(1), 20–27.
Kropski, J. A., Fremont, R. D., Calfee, C. S., & Ware, L. B. (2009). Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest, 135(6), 1440–1447.
Marchand-Adam, S., Plantier, L., Bernuau, D., Legrand, A., Cohen, M., Marchal, J., Soler, P., Lesèche, G., Mal, H., Aubier, M., Dehoux, M., & Crestani, B. (2005). Keratinocyte growth factor expression by fibroblasts in pulmonary fibrosis: poor response to interleukin-1beta. American Journal of Respiratory Cell and Molecular Biology, 32(5), 470–477.
Park, H. Y., Churg, A., Wright, J. L., Li, Y., Tam, S., Man, S. F., Tashkin, D., Wise, R. A., Connett, J. E., & Sin, D. D. (2013). Club cell protein 16 and disease progression in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 188(12), 1413–1419.
Tzouvelekis, A., et al. (2005). Serum biomarkers in interstitial lung diseases. Respiratory Research, 6, 78.
Elseweidy, M. M., Askar, M. E., Elswefy, S. E., & Shawky, M. (2018). Nephrotoxicity induced by cisplatin intake in experimental rats and therapeutic approach of using mesenchymal stem cells and spironolactone. Applied Biochemistry and Biotechnology, 184(4), 1390–1403.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission (1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; (2) drafted the work or revised it critically for important intellectual content; (3) approved the version to be published; and (4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Radwan, S.M., Ghoneim, D., Salem, M. et al. Adipose Tissue–Derived Mesenchymal Stem Cells Protect Against Amiodarone-Induced Lung Injury in Rats. Appl Biochem Biotechnol 191, 1027–1041 (2020). https://doi.org/10.1007/s12010-020-03227-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03227-8