Abstract
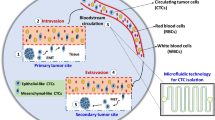
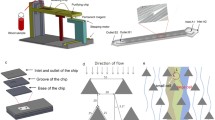
As a “liquid biopsy,” circulating tumor cell (CTC) enumeration with microfluidic chips has great significance in cancer prognosis. CTCs carry significant information as the original tumor. Integrated microfluidic chips are combining with affinity- and physical-based such as wave chip offers a new way to segregate CTCs. In this work, we further study capturing clinical applications of CTCs with wave chip. When cell suspension moves across the microposts array, CTCs squeeze out from narrow gaps organized by microposts. This movement renders CTCs to obtain a tilted velocity to fluid direction. This tilted velocity would direct CTCs to be captured by the smaller neighboring gaps next array. Simultaneously, interaction or friction time is longer due to barrier of modified microposts. These microposts would be effective for realizing binding of antigen and antibody. Therefore, both antibody-coated and physical-based isolations could be combined in isolating CTCs. Capture percentage concentrated on the first several arrays is shown theoretically and experimentally. Efficient capture could be obtained for artificial patient blood. Clinically, CTCs were tested positive for three metastatic human breast cancer patient samples. This wave chip is prospectively to be a valid tool for clinical enumeration of CTCs, carrying out anti-cancer drug assay.










Similar content being viewed by others
References
Pantel, K., Brakenhoff, R. H., & Brandt, B. (2008). Detection, clinical relevance and specific biological properties of disseminating tumor cells. Nature Reviews Cancer, 8(5), 329–340.
Mehlen, P., & Puisieux, A. (2006). Metastasis: a question of life or death. Nature Reviews Cancer, 6(6), 449.
Fehm, T., Morrison, L., Saboorian, H., Hynan, L., Tucker, T., & Uhr, J. (2002). Patterns of aneusomy for three chromosomes in individual cells from breast cancer tumors. Breast Cancer Research and Treatment, 75, 227.
Cristofabilli, M., Budd, G. T., Stopeck, A., Matera, J., Miller, M. C., Reuben, J. M., Doyle, G. V., Allard, W. J., Terstappen, L. W., & Hayes, D. F. (2004). Circulating tumor cells, disease progression, and survival in metastatic breast cancer. The New England Journal of Medicine, 351, 781.
Fan, T., Zhao, Q., Chen, J. J., Chen, W.-T., & Pearl, M. L. (2009). Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecologic Oncology, 112, 185.
Hsiao, Y.-S., Ho, B.-C., Yan, H.-X., Kuo, C.-W., Chueh, D.-Y., Yu, H.-H., & Chen, P. (2015). Integrated 3D conducting polymer-based bioelectronics for capture and release of circulating tumor cells. Journal of Materials Chemistry B, 3(25), 5103–5110.
Sollier, E., Go, D. E., Che, J., Gossett, D. R., O’Byrne, S., Weaver, W. M., Kummer, N., Rettig, M., Goldman, J., Nickols, N., McCloskey, S., Kulkarni, R. P., & Di Carlo, D. (2014). Size-selective collection of circulating tumor cells using Vortex technology. Lab on a Chip, 14(1), 63–77.
Attard, G., Swennenhuis, J. F., Olmos, D., Reid, A. H., Vickers, E., A'Hern, R., Levink, R., Coumans, F., Moreira, J., Riisnaes, R., Oommen, N. B., Hawche, G., Jameson, C., Thompson, E., Sipkema, R., Carden, C. P., Parker, C., Dearnaley, D., Kaye, S. B., Cooper, C. S., Molina, A., Cox, M. E., Terstappen, L. W. M. M., & de Bono, J. S. (2009). Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Research, 69(7), 2912–2918.
Hyun, K. A., & Jung, H. I. (2014). Advances and critical concerns with the microfluidic enrichments of circulating tumor cells. Lab on a Chip, 14(1), 45–56.
Nagrath, S., Sequist, L. V., Maheswaran, S., Bell, D. W., Irimia, D., Ulkus, L., Smith, M. R., Kwak, E. L., Digumarthy, S., & Muzikansky, A. (2007). Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature, 450(7173), 1235–1239.
Stott, S. L., Hsu, C.-H., Tsukrov, D. I., Yu, M., Miyamoto, D. T., Waltman, B. A., Rothenberg, S. M., Shah, A. M., Smas, M. E., Korir, G. K., Floyd, F. P., Gilman, A. J., Lord, J. B., Winokur, D., Springer, S., Irimia, D., Nagrath, S., Sequist, L. V., Lee, R. J., Isselbache, K. J., Maheswaran, S., Haber, D. A., & Toner, M. (2010). Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proceedings of the National Academy of Sciences of the United States of America, 107(43), 18392–18397.
Yoon, H. J., Kim, T. H., Zhang, Z., Azizi, E., Pham, T. M., Paoletti, C., Lin, J., Ramnath, N., Wicha, M. S., Hayes, D. F., Simeone, D. M., & Nagrath, S. (2013). Sensitive capture of circulating tumour cells by functionalised graphene oxide nanosheets. Nature Nanotechnology, 8(10), 735–741.
Li, N., Xiao, T., Zhang, Z., He, W., Cao, Y., Zhang, W., & Chen, Y. (2015). A 3D graphene oxide microchip and a Au-enwrapped silica nanocomposite-based supersandwich cytosensor toward capture and analysis of circulating tumor cells. Nanoscale, 7, 16354–16360.
Murlidhar, V., Zeinali, M., Grabauskiene, S., Ghannad-Rezaie, M., Wicha, M. S., Simeone, M., Ramnath, N., Reddy, R. M., & Nagrath, S. (2014). A radial flow microfluidic device for ultra-high-throughput affinity-based isolation of circulating tumor cells. Small, 10, 4895–4904.
Yoon, H. J., Shanker, A., Wang, Y., Kozminsky, M., Jin, Q., Palanisamy, N., Burness, M. L., Azizi, E., Simeone, D. M., Wicha, M. S., Kim, J., & Nagrath, S. (2016). Tunable thermal-sensitive polymer-graphene oxide composite for efficient capture and release of viable circulating tumor cells. Advanced Materials, 28(24), 4891–4897.
Shah, P., Kaushik, A., Zhu, X., Zhang, C., & Li, C. (2014). Chip based single cell analysis for nanotoxicity assessment. Analyst, 139(9), 2088–2098.
Shah, P., Zhu, X., Chen, C., Hu, Y., & Li, C. Z. (2014). Lab-on-chip device for single cell trapping and analysis. Biomedical Microdevices, 16(1), 35–41.
Zhang, Z., Xu, J., & Drapaca, C. (2018). Particle squeezing in narrow confinements. Microfluidics and Nanofluidics, 22, 120.
Zhang, Z., Drapaca, C., Chen, X., & Xu, J. (2017). Droplet squeezing through a narrow constriction minimum impulse and critical velocity. Physics of Fluids, 29, 072102.
Di Carlo, D., Irimia, D., Tompkins, R. G., & Toner, M. (2007). Continuous inertial focusing, ordering, and separation of particles in microchannels. Proceedings of the National Academy of Sciences, 104(48), 18892–18897.
Li, P., Mao, Z., Peng, Z., Zhou, L., Chen, Y., .Huang, P.-H., Truica, C. I., Drabick, J. J., El-Deiry, W. S., & Dao, M., (2015) Acoustic separation of circulating tumor cells. Proceedings of the National Academy of Sciences, 112(16), 4970–4975.
Zhang, Z., Xu, J., Hong, B., & Chen, X. (2014). The effects of 3D channel geometry on CTC passing pressure-towards deformability-based cancer cell separation. Lab on a Chip, 14(14), 2576–2584.
Ahmmed, S. M., Bithi, S. S., Pore, A. A., Mubtasim, N., Schuster, C., Gollahon, L. S., & Vanapalli, S. A. (2018). Multi-sample deformability cytometry of cancer cells. APL Bioeng., 2(3), 032002.
Shi, W., Wang, S., Maarouf, A., Uhl, C. G., He, R., Yunus, D., & Liu, Y. (2017). Magnetic particles assisted capture and release of rare circulating tumor cells using wavy-herringbone structured microfluidic devices. Lab on a Chip, 17(19), 3291–3299.
Liu, F., KC, P., Zhang, G., & Zhe, J. (2015). Microfluidic magnetic bead assay for cell detection. Analytical Chemistry, 88(1), 711–717.
Chen, H., Zhang, Z., Liu, H., Zhang, Z., Lin, C., & Wang, B. (2019). Hybrid magnetic and deformability based isolation of circulating tumor cells using microfluidics. AIP Advances, 9, 025023.
Chen, H., Zhang, Z., & Wang, B. (2018). Size and deformability-based isolation of circulating tumor cells with microfluidic chips and their clinical studies. AIP Advances, 8, 120701.
Chen, H., & Zhang, Z. (2018). An inertia-deformability hybrid CTC chip: design, clinical test and numerical study. Journal of medical devices, ASME, 12, 041004–041001.
Chen H., Cao B.,Sun B., Cao Y., Yang K.,& Lin Y(2017) Highly-sensitive capture of circulating tumor cells using micro-ellipse filters. Scientific Reports, 7:610.
Gogoi, P., Sepehri, S., Zhou, Y., Gorin, M. A., Paolillo, C., Capoluongo, E., Gleason, K., Payne, A., Boniface, B., Cristofanilli, M., Mogan, T. M., Fortina, P., Pienta, K. J., Handique, K., & Wang, Y. (2016). Development of an automated and sensitive microfluidic device for capturing and characterizing circulating tumor cells (CTCs) from clinical blood samples. PLoS One, 11(1), e0147400.
Tan, J., Sohrabi, S., He, R., & Liu, Y. (2018). Numerical simulation of cell squeezing through a micropore by the immersed boundary method. Proc.Inst. Mech. Eng., Part C, 232(3), 502–514.
Mohamed, H., Murray, M., Turner, J. N., & Caggana, M. (2009). Isolation of tumor cells using size and deformation. Journal of Chromatography. A, 1216(47), 8289–8295.
Ahmmed, S. M., Suteria, N. S., Garbin, V., & Vanapalli, S. A. (2018). Hydrodynamic mobility of confined polymeric particles, vesicles, and cancer cells in a square microchannel. Biomicrofluidics, 12(1), 014114.
Sarioglu, F., Aceto, A., Kojic, N., Donaldson, M. C., Zeinali, M., Hamza, B., Engstrom, A., Hamza, B., Zeigstrom, A., Zhu, H., Sundaresan, T. K., Miyamoto, D. T., Luo, X., Bardia, A., Wittner, B. S., Ramaswamy, S., Shiodae, T., Ting, D. T., Stott, S. L., Kapur, R., Maheswaran, S., Haber, D. A., & Toner, M. (2015). A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nature Methods, 12(7), 685–691.
Kim, T. H., Yoon, H. J., Stella, P., & Nagrath, S. (2014). Cascaded spiral microfluidic device for deterministic and high purity continuous separation of circulating tumor cells. Biomicrofluidics, 8, 064117.
Todenhofer, T., Park, E. S., Duffy, S., Deng, X., Jin, C., Abdi, H., Ma, H., & Black, P. C. (2016). Microfluidic enrichment of circulating tumor cells in patients with clinically localized prostate cancer. Urologic oncology, 34(11), 483.e9–483.e16.
Kim, M. S. (2012). SSA-MOA: a novel CTC isolation platform using selective size amplification (SSA) and a multi-obstacle architecture (MOA) filter. Lab on a Chip, 12(16), 2874–2880.
Hosseini, S. A., Abdolahad, M., Dahmardeh, M., Gharooni, M., Abiri, H., Alikhani, A. S., Mohajerzadeh, A. S., & Mashinchian, O. (2016). Nanoelectromechanical chip (NELMEC) combination of nanoelectronics and microfluidics to diagnose epithelial and mesenchymal circulating tumor cells from leukocytes. Small, 12(7), 883–891.
Li, D., Zhang, Y., Li, R., Guo, J., Wang, J., & Tang, C. (2015). Selective capture and quick detection of targeting cells with SERS-coding microsphere suspension chip. Small, 11, 2200.
Chung, J., Issadore, D., Ullal, A., Lee, K., Weissleder, R., & Lee, H. (2013). Rare cell isolation and profiling on a hybrid magnetic/size-sorting chip. Biomicrofluidics, 7, 9.
Chen, H., Chen, H., Lin, Y., & Zhang, J. (2017). Combination of antibody-coated, physical-based microfluidic chip with wave-shaped arrays for isolating circulating tumor cells. Biomedical Microdevices, 19(3), 66.
Funding
This research work was supported by the Anhui Natural Science Foundation of China (1908085MF197) and Postdoctoral Research Funding (2014M550794).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
Patient blood samples were supplied by Longhua Hospital Affiliated to Shanghai Medical University under approval.
Informed Consent
The manuscript is approved by all authors for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H. Capturing and Clinical Applications of Circulating Tumor Cells with Wave Microfluidic Chip. Appl Biochem Biotechnol 190, 1470–1483 (2020). https://doi.org/10.1007/s12010-019-03199-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03199-4